August 14, 2018
The Impact of DPP-4 on Active GLP-1 Preanalytical Variability
Active glucagon-like peptide-1 (active GLP-1) is an incretin hormone known for its pivotal role in the regulation of insulin and glucagon secretion1,2. However, research has demonstrated that the accurate quantification of active GLP-1 is difficult to achieve and has led to the widespread inability to compare data across studies3,4,5. It has been determined that the DPP-4 mediated degradation of active GLP-1 is one of the main sources of active GLP-1 preanalytical variability in samples3,4,5. Research has shown that the impact of DPP-4 on active GLP-1 preanalytical variability can be reduced when proper active GLP-1 sample handling is performed3,4,5.
DPP-4 Mediated Degradation of Active GLP-1
Active GLP-1 is secreted from intestinal L-cells postprandially. The hormone is then rapidly cleaved and degraded by the enzyme dipeptidyl peptidase-4 (DPP-4), resulting in a 1½ - 2minute half-life in vivo1,6. DPP-4 cleaves active GLP-1 (7-36) amide and active GLP-1 (7-37) non-amide between the second and third N-terminal amino acid residues, forming GLP-1 metabolites4. Analysis by mass spectrometry has shown that in human EDTA plasma samples spiked with both forms of active GLP-1, concentrations of the active forms decrease rapidly over time as concentrations of GLP-1 metabolites increase4. Therefore, the presence of DPP-4 greatly impacts the stability of the hormone, making accurate quantification of active GLP-1 extremely difficult4.The Impact of DPP-4 on Active GLP-1 Preanalytical Variability
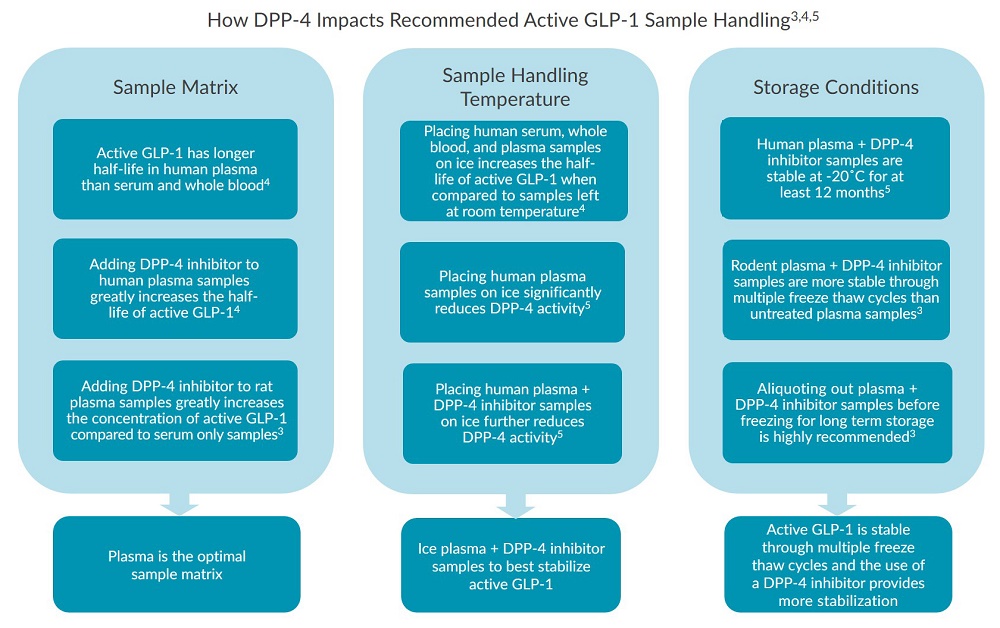
DPP-4 activity influences how preanalytical human and rodent samples must be handled in order to accurately measure active GLP-13,4,5. The effects of DPP-4 on active GLP-1 concentrations have been tested by comparing the recovery of active GLP-1 in samples which have been collected in the presence or absence of DPP-4 inhibitors under various conditions3,4,5. When quantifying active GLP-1, research has shown that the presence of DPP-4 impacts the following aspects of sample handling3,4,5:- Optimum sample matrix
- Sample handling temperature
- Storage conditions
Recommendations for the Accurate Quantification of Active GLP-1
Although active GLP-1 can be a very unstable hormone, proper sample handling techniques and storage conditions can be employed to reduce the impact of DPP-4 on active GLP-1 preanalytical variability. Ultimately, proper active GLP-1 sample handling requires the addition of DPP-4 inhibitors which prevent the DPP-4 mediated degradation of active GLP-13,4,5. Research also indicates that plasma is the optimal sample matrix to use when quantifying active GLP-1 due to its lower amounts of cell surface and soluble forms of DPP-43. Plasma samples should be kept on ice to slow the enzymatic activity of DPP-4 and, therefore, increase the half-life and stability of active GLP-14,5. Furthermore, evidence shows that active GLP-1 is relatively stable after multiple freeze thaw cycles if samples are collected in the presence of DPP-4 inhibitors and kept on ice upon thawing3,4,5.
Conclusion
The accurate quantification of active GLP-1 can be achieved in both human and rodent models by following appropriate sample handling methods which prevent the DPP-4 mediated degradation of active GLP-13,4,5. By doing so, researchers can reduce the impact of DPP-4 on active GLP-1 preanalytical variability3,4,5. As a result, proper active GLP-1 sample handling will enable diabetes researchers to more easily compare important data across studies which could eventually lead to innovative scientific breakthroughs. Download our Active GLP-1 (7-36) amide Sample Collection and Handling Guide to learn about the impact of DPP-4 on active GLP-1 preanalytical variability. DownloadReferences
- Holst (2007). The Physiology of Glucagon-like Peptide 1; Physiol Rev. 2007 Oct;87(4):1409-39. PMID: 17928588.
- Sandoval and D’Alessio (2015). Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015 Apr;95(2):513-48. doi: 10.1152/physrev.00013.2014. PMID: 25834231.
- Bielohuby, et al (2012). A guide for measurement of circulating metabolic hormones in rodents: Pitfalls during the pre-analytical phase. Mol Metab. 2012 Aug 9;1(1-2):47-60. PMID: 24024118
- Yi, et al (2015). Degradation and stabilization of Peptide hormones in Human Blood Specimens. PLoS One. 2015 Jul 29;10(7):e0134427. PMID: 26222180.
- Wewer Albrechtsen, et al (2015). Stability of glucagon-like peptide 1 and glucagon in human plasma. Endocrine Connections, 4:50-57. PMID: 25596009.
- Deacon, et al (1995). Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo; J Clin Endocrinol Metab. 1995 Mar; 80(3):952-7. PMID: 7883856.

