August 28, 2018
The Generation and Function of Active GLP-1
The Generation and Function of Active GLP-1
Active glucagon-like peptide-1 (GLP-1) is a member of the incretin hormone family and is generated as a result of post translational modifications during proglucagon processing1,2. Active GLP-1 plays a key role in the incretin effect and is vital for maintaining glucose homeostasis1,2. As such, both the generation and function of active GLP-1 have become important aspects of type 2 diabetes (T2D) research2.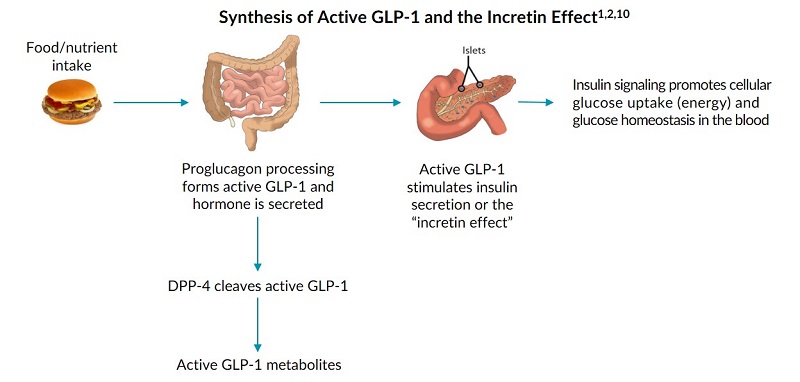
Proglucagon Processing
The process of generating active GLP-1 is referred to as proglucagon processing. This begins with the synthesis of preproglucagon (PPG), the single precursor to all of the proglucagon peptides3. The synthesis of preproglucagon is highly regulated and occurs in the gut, brain, and pancreas3-7. A post translational modification (PTM) enzymatically cleaves preproglucagon into proglucagon3.Active GLP-1 Forms Generated by the Gut and Brain
In the gut and brain, proglucagon is then further processed into various proglucagon peptides, including active GLP-18,9. Two active forms of GLP-1 are generated in the gut and brain: active GLP-1 (7-36) amide and active GLP-1 (7-37) non-amide10. However, research indicates that active GLP-1 (7-36) amide is the most predominant form in circulation and can provide an accurate representation of active GLP-1 levels11, 12.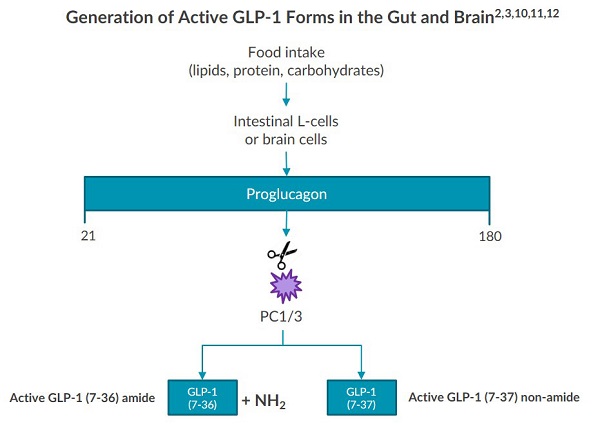
The Incretin Effect and Active GLP-1
Research indicates that active GLP-1 has many physiological effects on the body such as the incretin effect. Following the intake of nutrients, the beginning of the incretin effect is marked by rising levels of active GLP-1 which promote the synthesis and release of insulin 1,2,4. This cyclical pathway is vital to maintain energy homeostasis 1,2,4.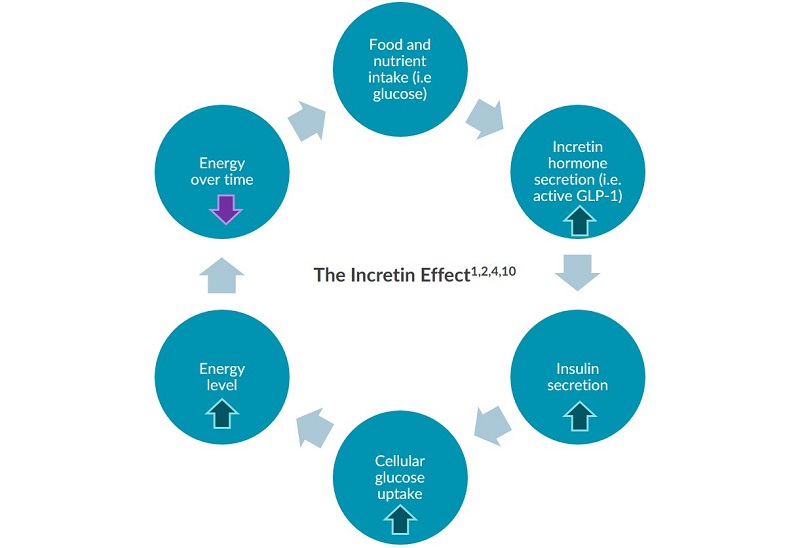
The Generation of Active GLP-1 Metabolites and Isoforms
Multiple metabolites and isoforms of active GLP-1 exist in circulation. The enzyme dipeptidyl peptidase 4 (DPP-4) cleaves active GLP-1 (7-36) amide and (7-37) non-amide after secretion from the gut and brain, forming two GLP-1 metabolites8,10,12,13. Additionally, research indicates that the pancreas can secrete two isoforms of active GLP-1 under metabolic stress12,13,14.Diabetes Research and GLP-1
Active GLP-1 is highly conserved across mammalian species, allowing investigators to study the hormone across different model organisms such as rodents and humans for over 20 years2,10,15. Researchers can utilize ELISAs in their labs to accurately measure active GLP-1. Early studies discovered a reduced incretin effect in individuals with type 2 diabetes following oral glucose intake16,17,18. More recently, researchers are trying to understand the connection between active GLP-1 and sweet taste transmission to uncover new treatments for diabetes and obesity19,20,21,22.Conclusion
Active GLP-1 is a gut hormone derived as a result of proglucagon processing1,2. Once active GLP-1 is secreted, the enzyme DPP-4 quickly cleaves the majority of the hormone10 and the remaining active GLP-1 enters circulation. Active GLP-1 then flows to peripheral organs where it stimulates the incretin effect1,2,10. The unique generation and function of active GLP-1 has influenced growing interest in the hormone, and its reputation as an important biomarker in type 2 diabetes research16,21,23. Download our eBook to learn about the generation and function of active GLP-1 and why both are important aspects of type 2 diabetes research. Download eBookReferences
- Pacific Biomarkers (2013). Incretins and Gastrointestinal Hormones: Pathophysiology and Pre-analytical Considerations. White Paper. Pacbio.com.
- MacDonald, et al (2002). The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes December; vol. 51 no. suppl 3 S434-S442. PMID: 12475787.
- Sandoval and D’Alessio (2015). Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015 Apr;95(2):513-48. doi: 10.1152/physrev.00013.2014. PMID: 25834231.
- Kim and Egan (2008). The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharmacol Rev. 2008 Dec;60(4):470-512. PMID: 19074620.
- Jin (2008). Mechanisms underlying proglucagon gene expression. J Endocrinol. 2008 Jul;198(1):17-28. doi: 10.1677/JOE-08-0085. PMID: 18577568.
- Shao, et al (2013). The Wnt Signaling Pathway Effector TCF7L2 Controls Gut and Brain Proglucagon Gene Expression and Glucose Homeostasis. 2013 Mar; 62(3): 789–800. PMID: 22966074.
- Trapp and Cork (2015). PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol. 2015 Oct 15;309(8):R795-804. PMID: 26290108.
- Holst (2007). The Physiology of Glucagon-like Peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID: 17928588.
- Blanco, et al (2014). Biochemical and cell biological properties of the human prohormone convertase 1/3 Ser357Gly mutation: a PC1/3 hypermorph. Endocrinology. 2014 Sep;155(9):3434-47. doi: 10.1210/en.2013-2151. PMID: 24932808.
- Janssen, et al (2012). Review article: a comparison of glucagon-like peptides 1 and 2. Aliment Pharmacol Ther. 2013 Jan;37(1):18-36. doi: 10.1111/apt.12092. PMID: 23121085.
- Nadkarni, et al (2014). Regulation of Glucose Homeostasis by GLP-1. Prog Mol Biol Transl. 2014;121:23-65. PMID: 24373234.
- Bak, et al (2014). Specificity and sensitivity of commercially available assays for glucagon-like peptide 1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes Obes Metab. 2014 Nov;16(11):1155-64. doi: 10.1111/dom.12352. PMID: 25041349.
- Whalley, et al (2011). Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol. 2011 Oct;211(1):99-106. doi: 10.1530/JOE-11-0094. PMID: 21795304.
- Tateishi, et al (1994). Glucagon-like peptide-1 (GLP-1) molecular forms in human pancreatic endocrine tumors resemble those in intestine rather than pancreas. Diabetes Res Clin Pract.1994 Aug;25(1):43-9. PMID: 7835211.
- Tornehave, et al (2008). Expression of the GLP-1 Receptor in Mouse, Rat, and Human Pancreas. J Histochem Cytochem. 2008 Sep; 56(9): 841–851. PMCID: PMC2516959.
- Nauck, et al (1986). Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia.1986 Jan;29(1):46-52. PMID: 3514343.
- Knop, et al (2007). Reduced Incretin Effect in Type 2 Diabetes: Cause or consequence of the diabetic state? 2007 Aug;56(8):1951-9. PMID: 17513701.
- Godoy-Matos (2014). The role of glucagon on type 2 diabetes at a glance. Diabetol Metab Syndr. 2014 Aug 24;6(1):91. doi: 10.1186/1758-5996-6-91. PMID: 25177371.
- Bagley (2018). Taster’s Choice: The Endocrinology of the Tongue. Endocrinology News. March 2018.
- Takai, et al (2015). Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015 Jun;29(6):2268-80. PMID: 25678625.
- Santa-Cruz Calvo and Egan (2015). The Endocrinology of Taste Recptors. Nat Rev Endocrinol. 2015 Apr;11(4):213-27. PMID: 25707779.
- Ten Kulve, et al (2016). Endogenous GLP1 and GLP1 analogue alter CNS response to palatable food consumption. J Endocrinol. 2016 Apr;229(1):1-12. PMID: 26769912.
- Holst, et al (2011). Loss of Incretin Effect Is a Specific, Important, and Early Characteristic of Type 2 Diabetes. Diabetes Care. 2011 May;34 Suppl 2:S251-7. doi: 10.2337/dc11-s227. PMID: 21525464.