January 12, 2016
Simplify Stool Sample Processing
Introduction
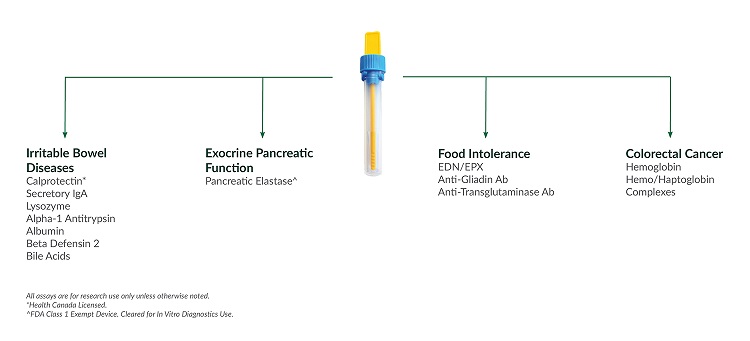
We understand the challenges facing lab managers and directors who need to continuously improve workflow efficiency and implement new tests and technologies to be competitive. Our unique extraction method can be used with a portfolio of targeted stool markers. This allows your lab to simplify stool sample processing time by up to 86% by extracting one sample for up to 13 stool-based biomarkers.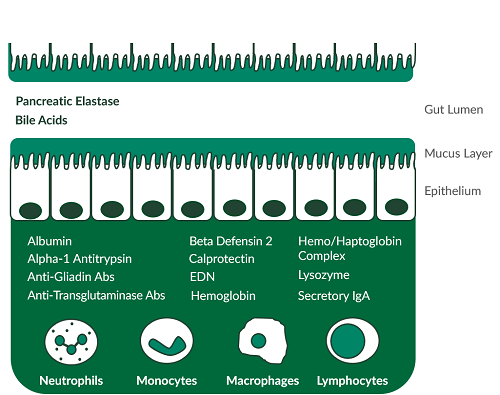
Simplify Stool Sample Processing Time
Sample Collection
Stool samples can uncover valuable information as to what is occurring in the gastrointestinal system. Unlike other laboratory tests, stool samples are commonly collected at home by the patient. However, patients find the collection of stool to be difficult, unpleasant and unhygienic to perform. Many patients take a sample directly from the toilet basin which is not only difficult, but may cause potential measurement errors due to the loss of content from surrounding water or contamination with toilet sanitizers and additives. The EasySampler® Stool Collection Kit is a user-friendly and hygienic stool sample collection method and includes:- 1 EasySampler® collection hat (paper portion)
- 1 pair of disposable gloves
- 2 collection spoons
- 1 leak-proof primary receptacle with screw cap
- 1 leak-proof secondary receptacle with absorbent material and screw cap
- 1 padded envelope marked for mailing biological material

Stool Extraction and Processing
The Easy Stool Extraction Device is an FDA Class I Exempt tool that can be used across four comprehensive gastroenterology ELISA testing panels comprised of 13 different analytes. The device allows lab technicians to replace manual weighing with one simple step. A grooved plastic wand is dipped into a stool sample in multiple areas and placed inside a vial pre-filled with a universal extraction buffer. This standardized device accurately measures 15 mg of stool and extracts the sample in 1.5 mL of extraction buffer, yielding a final dilution of 1:100. The resulting extract can be placed directly on automated platforms and run across up to 13 different assays. • Increase efficiency by minimizing stool sample extractions • Allow for additional sample processing and other activities • Offer more comprehensive gastroenterology testing panels • Improve clinical outcomes with faster turnaround timeDilution and Stability of Stool Samples
Our panel of stool-based ELISAs may require a simple secondary dilution of extracted stool sample, and biomarkers in the extracted stool samples have a range of stabilities. Additionally, sometimes not all biomarkers from a single stool sample are determined on the same day; therefore, it might be necessary to store the stool extract. The following reference table outlines the different secondary extracted stool sample dilutions, stabilities, and maximum freeze thaw cycles for our panel of stool-based ELISAs: [caption id="attachment_4276" align="alignnone" width="750"]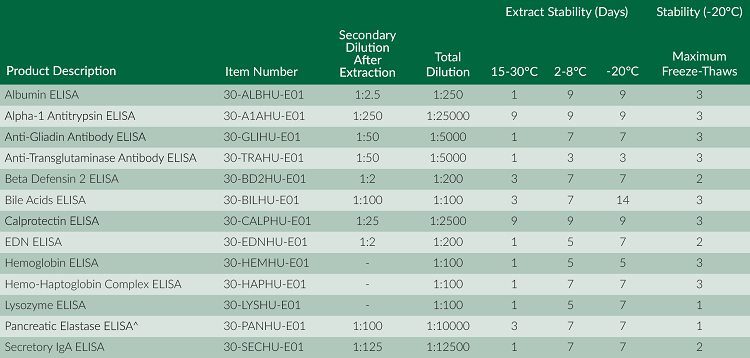 All assays are for Research Use Only unless otherwise noted. ^FDA Class I Exempt Device. Cleared for In Vitro Diagnostics Use.[/caption]
All assays are for Research Use Only unless otherwise noted. ^FDA Class I Exempt Device. Cleared for In Vitro Diagnostics Use.[/caption]
Assays for the Study of Inflammatory Bowel Diseases: IBD and IBS
IBD is a chronic disease with forms involving the lower bowel parts or the entire GI tract, causing symptoms like abdominal pain, diarrhea, fever and weight loss. An estimated two million people in North America suffer from IBD seemingly caused by an overactive mucosal immune system1. Crohn’s disease and ulcerative colitis are the major groups of inflammatory conditions that make up IBD and are incurable, serious and chronic organic diseases of the intestinal tract. The symptoms are distressing, embarrassing and even debilitating. IBS is a non-organic functional disorder. It expresses similar symptoms to IBD such as cramping, bloating, diarrhea, and constipation. An estimated thirty million people in North America are affected by IBS symptoms, resulting in over 3.5 million physician visits annually and accounting for roughly 30% of visits to gastroenterologists2. Researchers have identified several key stool biomarkers which demonstrate the potential to aid in the differentiation between IBD and IBS.Assays for the Study of Exocrine Pancreatic Function
Exocrine pancreatic function encompasses the digestive functions of the pancreas such as generating enzymes to breakdown starches, fats and proteins. Pancreatic insufficiency can be the result of a number of different ailments such as pancreatic cancer and Crohn’s disease3. Pancreatic elastase is one of the enzymes generated by the pancreas and is mainly bound to bile salts during intestinal passage and is not degraded3. The concentration of the enzyme in stool samples reflects the secretory capacity of the pancreas. Stool pancreatic elastase levels can define exocrine pancreatic function, with low levels indicating the presence of pancreatic insufficiency3.Assays for the Study of Celiac Disease
Although sometimes confused with food allergies, food intolerance is a different health issue. An individual with a food intolerance maybe able to still continue to consume the food they have the issue with. Food intolerance can be caused by IBS, celiac disease, food poisoning, or the inability to produce specific digestive enzymes4. Celiac disease can be researched with the following stool-based ELISAs:Assays for the Study of Colorectal Cancer
Colorectal cancer starts in either the colon or the rectum. Almost 100,000 new cases of colorectal cancer are estimated to develop in the United States in 20175. Research on colorectal cancer has been focusing on methods for the early detection of the disease6. Stool biomarkers being investigated in colorectal cancer include:Gastroenterology Testing Panel Guide
Stool sample collection, extraction, and processing is simple and cost effective when using both the EasySampler® Stool Collection Kit and Easy Stool Extraction Device. One 15 mg stool sample allows for the analysis of 13 different biomarkers that can be used to investigate gastroenterology research areas such as inflammatory bowel diseases, exocrine pancreatic function, celiac disease, and colorectal cancer. Download our gastroenterology testing guide to learn more about these biomarkers and how you can simplify stool sample processing time in your lab. Download Testing Guide
Download Testing Guide
References
- Crohn's & Colitis Foundation of America (CCFA). (2011). Facts about Inflammatory Bowel Diseases. CCFA.org.
- International Foundation for Functional Gastrointestinal Disorders (IFFGD). (2013). What is IBS? aboutIBS.org.
- Medscape. (2014). Exocrine Pancreatic Insufficiency. Medscape.com.
- Mayo Clinic. (2014). What's the difference between a food intolerance and food allergy? Mayoclinic.org.
- American Cancer Society. (2017). Key Statistics for Colorectal Cancer. Cancer.org.
- Goldshtein et al. (2010). Variations in hemoglobin before colorectal cancer diagnosis. Eur. J. Cancer Prev., 19(5). 342-4. PMID: 20543703.