May 19, 2022
Understanding Exocrine Pancreatic Insufficiency
Pancreatic Elastase (PE) Testing for Determination of Exocrine Pancreatic Insufficiency (EPI)
 Advantages of ALPCO PE Testing for Determination of EPI
Advantages of ALPCO PE Testing for Determination of EPI
• No interference from PERT
• Single sample collection without dietary restriction
• Detection of both CELA3A and CELA3B isoforms
• Compatibility with current clinical cut-offs
• FDA Class I Device. For In Vitro Diagnostic Use.
• Accurate and reproducible
• Validated for use with extraction device, reducing the need for manual weighing
Understanding Exocrine Pancreatic Insufficiency
Exocrine pancreatic insufficiency (EPI) involves the malabsorption of fats, proteins, and carbohydrates due to inadequate delivery of pancreatic enzymes that normally break down those fuels in the intestines.1 Symptoms of EPI can include bloating, abdominal pain, steatorrhea, vitamin deficiency, and weight loss.2 Particularly in children, delayed diagnosis of EPI can cause impaired growth. Early detection and treatment can minimize symptoms and prevent vitamin deficiency and malnutrition.3Causes of EPI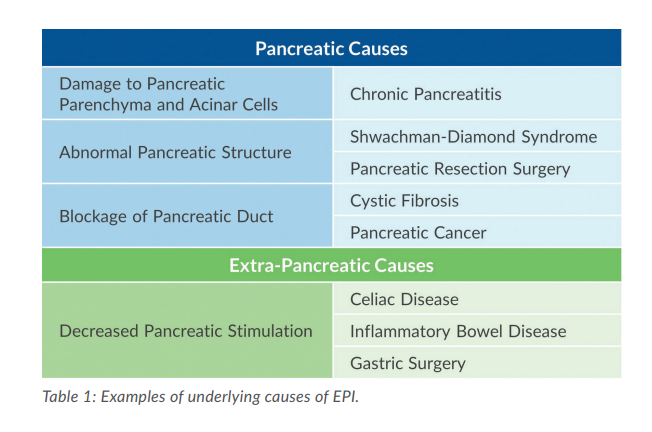
A wide range of diseases and disorders can lead to insufficient production, secretion, and/or function of pancreatic enzymes. The table (right) outlines some of the underlying pancreatic and extra-pancreatic causes of EPI, including chronic pancreatitis, cystic fibrosis, pancreatic cancer, and intestinal diseases that can affect signaling.3,4
Methods for Diagnosing Exocrine Pancreatic Insufficiency
A variety of methods are currently used to assist with the diagnosis of EPI and their utility varies based on the underlying cause. Direct tests for pancreatic enzyme production are believed to be most effective for detection of mild pancreatic insufficiency, but they will not detect EPI caused by a decrease in intestinal signaling.3,4 Direct tests are also invasive, expensive, and rarely performed in the US. Some tests, including chymotrypsin and fecal fat, require a special diet and / or cessation of pancreatic enzyme replacement therapy (PERT) and daily stool collection. Issues with patient compliance often affect the accuracy of results. In addition, indications other than EPI, such as bile acid deficiency and diseases of the proximal small intestine, can cause high fecal fat levels.2,4-6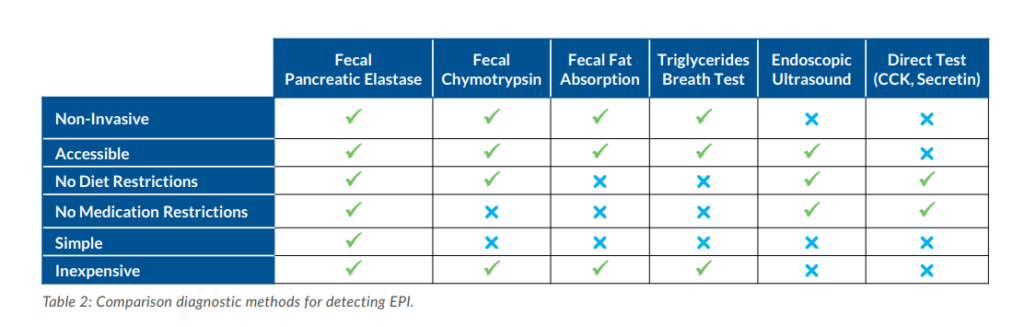
Detection of EPI with Fecal Pancreatic Elastase
Pancreatic elastase (PE or PE-1) refers to a family of chymotrypsin-like elastases (CELAs), digestive proteases produced by acinar cells in the pancreas. PE is highly stable in the intestinal tract and the concentration of pancreatic elastase in stool correlates well with overall pancreatic enzyme output.7,8ALPCO’s Simple, Accurate Test for PE and EPI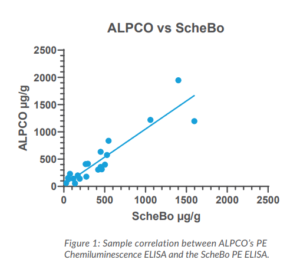
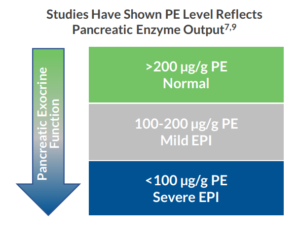
Several commercial assays are available for the measurement of pancreatic elastase in stool. Research has shown that PE levels between 100 and 200 µg/g indicate moderate EPI and levels below 100 µg/g indicate severe EPI.7,9 The Pancreatic Elastase Chemiluminescence ELISA* from ALPCO is a 510(k) exempt device that aids in the diagnosis of exocrine pancreatic insufficiency. This assay is calibrated to the original PE assay used in the early investigation of EPI.
Pancreatic Elastase Assay Specificity
Of five CELA isoforms of pancreatic elastase, only three are expressed in humans as active proteases. Both the CELA3A and CELA3B isoforms can be measured in stool and are detected by most commercially available assays to varying degrees.10 Using a proprietary CELA3B-specific assay alongside a CELA3A + CELA3B assay, ALPCO scientists measured the CELA3A and CELA3B isoforms in fecal samples from 32 apparently healthy individuals. The proportion of CELA3A to CELA3B isoforms varied significantly (Figure 3), indicating sufficient measurement of both isoforms is required for a complete assessment of PE production and proper determination of EPI. Detection of both isoforms of PE has bee demonstrated for multiple lots of the Pancreatic Elastase Chemiluminescence ELISA.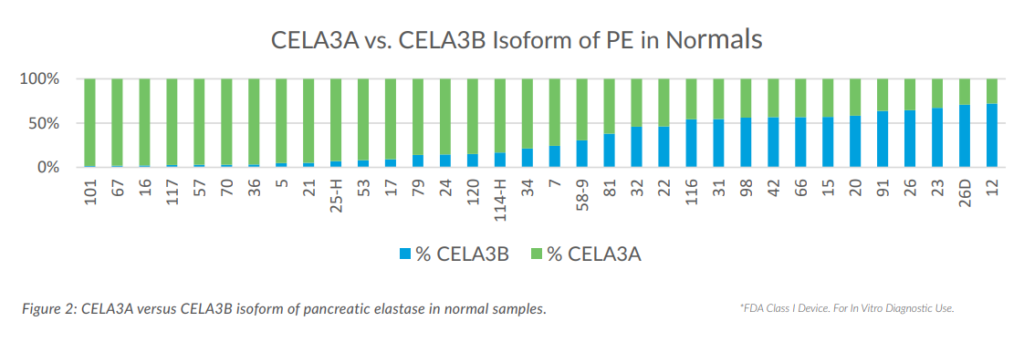
Pancreatic Enzyme Secretion in Digestion
While pancreatic disease reduces pancreatic enzyme secretion in all phases of digestion, insufficient signaling in the gastric and intestinal phases can also cause pancreatic enzyme insufficiency. This can lead to malabsorption of nutrients, deficiency in fat-soluble vitamins, and malnutrition. Tests for fecal pancreatic elastase can detect EPI caused by disorders affecting the production of pancreatic enzymes (damage to pancreatic parenchyma), the release of pancreatic enzymes (blockage of pancreatic duct), and the stimulation of enzyme secretion (intestinal disorders).11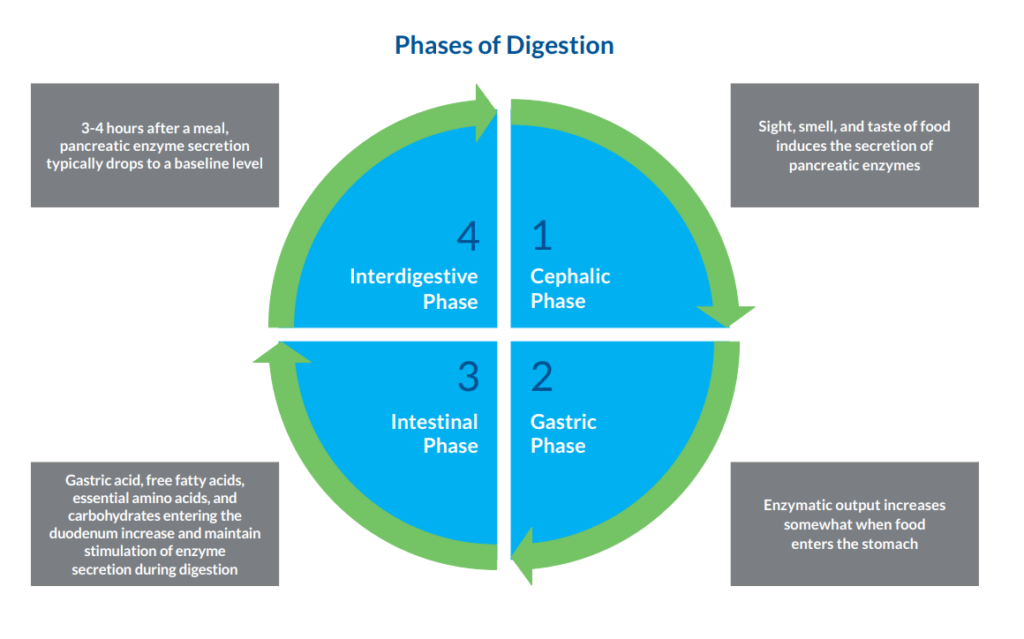
The Role of Diet in EPI Detection and Treatment
Digestive enzymes produced by salivary glands and the stomach enable the digestion of medium-chain fatty acids. Pancreatic lipase is specifically required for the hydrolysis of essential long-chain polyunsaturated fatty acids required for proper nutrition.12 In healthy individuals, high fat and high protein diets have been shown to increase pancreatic enzyme outputs. Long-chain fatty acids and certain essential amino acids produce a greater increase in pancreatic enzymes than other nutrients.11 Significant changes in diet can potentially affect pancreatic enzyme levels and pancreatic elastase measurements over time, particularly in normal patients or those suspected of mild insufficiency. A patient’s diet, symptoms, and test results should be considered together when diagnosing pancreatic insufficiency and determining a patient’s need for pancreatic enzyme replacement therapy.
Schedule a Pancreatic Elastase Chemiluminescence ELISA* Evaluation Today!
Please call (800) 592-5726 or email [email protected] to:
• Find a testing lab running the assay
• Implement the assay in your lab
• Request a trial kit or a presentation on GI biomarkers
*FDA Class I Device. For In Vitro Diagnostic Use.
References
- Jawaid and Forsmark (2019). Exocrine pancreatic insufficiency following acute pancreatitis: True association or EPI phenomenon? Digestive Diseases and Sciences. 64:1731-1733.
- Capurso, et al (2019). Exocrine pancreatic insufficiency: prevalence, diagnosis, and management, Clinical and Experimental Gastroenterology.12:129-139.
- Sankararaman, et al (2019). Management of exocrine pancreatic insufficiency in children. Nutrition in Clinical Practice. 34(1)S27-S42.
- Drewes (2013). Diagnosis and treatment of pancreatic exocrine insufficiency. World Journal of Gastroenterology. 19(42): 7258-7266.
- Singh, et al (2017). Less common etiologies of exocrine pancreatic insufficiency. World Journal of Gastroenterology. 23(39): 7059-7076.
- Azer, et al [Updated 2020 May 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541055/.
- Lohr, et al ( 2016). Clinical and laboratory diagnosis of chronic pancreatitis. Pancreapedia. DOI: 10.3998/panc.2016.14.
- Meyts, et al (2002). Variability of fecal pancreatic elastase measurements in cystic fibrosis patients. Journal of Cystic Fibrosis. 2002:265-268.
- Toth, et al (2017). Detection of human elastase isoforms by the ScheBo Pancreatic Elastase 1 Test. Am J Physiol Gastrointest Liver Physiol.312(6), G606-G614.
- Parniczky, et al (2016). Genetic analysis of human chymotrypsin-like elastases 3A and 3B (CELA3A and CELA3B) to assess the role of complex formation between proelastases and procarboxypeptidases in chronic pancreatitis. Int’l Journal of Molecular Sciences. 17: 2148. doi:10.3390/ijms17122148.
- Keller and Layer (2005). Human pancreatic exocrine response to nutrients in health and disease. Gut. 54(Suppl VI):vi1–vi28.
- Freedman (2017). Options for Addressing Exocrine Pancreatic Insufficiency in Patients Receiving Enteral Nutrition Supplementation. Am J Manag Care. 2017;23:-S0. https://www.ajmc.com/view/options-for-addressing-exocrine-pancreatic-insufficiency-in-patients-receiving-enteral-nutrition-supplementation-article
Pancreatic Elastase Chemiluminescence ELISA
View Product Listing
Interested in walk-away automation for your PE assays? Learn about our PE flash chemiluminescence assay for the KleeYa platform.
Learn More
Brochure: Understanding Exocrine Pancreatic Insufficiency
Download