Managing Calprotectin as a Send Out?
While sending out for calprotectin testing offers some cost and convenience advantages when volumes are very low, bringing the process in-house allows for the opportunity to control costs, turnaround time, and quality. Considering costs and potential revenue, the volume at which in-house calprotectin testing makes sense may be lower than you think.
How to Increase Profitability with In-house Calprotectin Testing from ALPCO
If your lab is sending calprotectin out for analysis at a third-party laboratory, testing just seven samples at a time in-house may reduce your net cost per sample. The more samples currently being outsourced, the more money your lab can save by analyzing new samples in-house. ALPCO simplifies the addition of new tests by assisting with reagent supply, well-characterized samples, assay training, workflow optimization, and validation testing. Furthermore, if you see an increase in volume on the horizon, in-house testing can help you to capture more testing revenue. Transitioning to in-house calprotectin testing with ALPCO has several advantages for your lab:
-
Cost savings
-
Higher potential for revenue growth
-
Faster turn-around time and associated savings through quicker diagnosis
-
Control over data quality
-
Implementation and validation support
-
Reduction in false positive rate for IBD determination
Fecal calprotectin tests are non-invasive tools that assist with the diagnosis of inflammatory bowel disease (IBD) and the differentiation of inflammatory bowel disease from irritable bowel syndrome (IBS). ALPCO offers the most clinically accurate 510(k)-cleared calprotectin test on the market today. When compared to other calprotectin tests, our assay reduces false positives for IBD from 20 – 33% to just 7.5%.
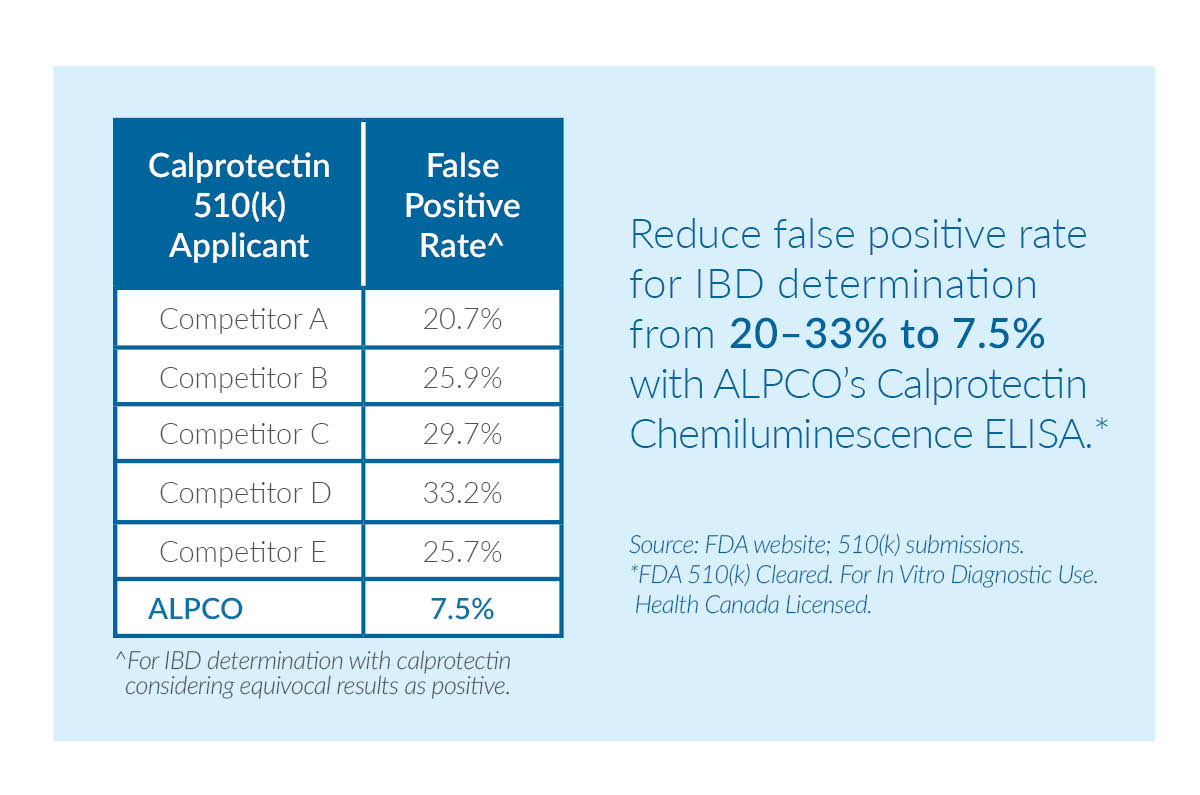
Figure 1: When compared to five different calprotectin tests, ALPCO's assay has the lowest false positive rate for IBD determination.
Implementation and Validation Assistance
ALPCO makes your switch simple. Our convenient Validation Assistance Program enables your lab to quickly get up and running with the Calprotectin Chemiluminescence ELISA. We provide validation kits, stool extraction devices, a well-characterized sample panel, workflow optimization, and in-lab support. After the assay validation is completed, ALPCO will continue to support your team.
Learn How In-house Calprotectin Testing Can Benefit Your Lab
Complete the form to learn more about bringing calprotectin testing in-house and request a free evaluation kit.
In-house Calprotectin Testing Brochure

