March 6, 2018
Researching the Role of Gut Permeability in NAFLD
[playlist images="false" artists="false" tracklist="false" ids="4600"]
A growing amount of research demonstrates that communication between the gut microbiota and liver is vital for the regulation of energy metabolism1,2. As a result, scientists are further exploring the relationship between the gut microbiota and gut-liver crosstalk to better understand metabolic diseases such as non-alcoholic fatty liver disease (NAFLD)3. Recent findings indicate that the gastrointestinal (GI) protein zonulin may influence the role of gut permeability in NAFLD4,5,6. Zonulin may also be involved in the pathogenesis of NAFLD and is considered a potential biomarker for the disease4,5,6.
 The gut microbiota is a unique collection of microorganisms vital for proper gut-liver crosstalk and maintaining homeostasis1,2. Biomolecules produced by the liver and microbiota aid in gut-liver crosstalk and support various metabolic functions2,7. Although the gut microbiota provides many benefits to humans, changes in its composition can lead to imbalances in SCFA and BAs, inflammation, and changes to gut permeability2,3,6,13. As such, studies show increased zonulin levels in NAFLD, which may indicate its involvement in the 4,5,6. More research is necessary, however, to fully understand the relationship between the gut microbiota and gut-liver crosstalk, as well as the role of gut permeability in NAFLD.
The gut microbiota is a unique collection of microorganisms vital for proper gut-liver crosstalk and maintaining homeostasis1,2. Biomolecules produced by the liver and microbiota aid in gut-liver crosstalk and support various metabolic functions2,7. Although the gut microbiota provides many benefits to humans, changes in its composition can lead to imbalances in SCFA and BAs, inflammation, and changes to gut permeability2,3,6,13. As such, studies show increased zonulin levels in NAFLD, which may indicate its involvement in the 4,5,6. More research is necessary, however, to fully understand the relationship between the gut microbiota and gut-liver crosstalk, as well as the role of gut permeability in NAFLD.
Functions and Benefits of Gut Microbiota
The human gut microbiota is a unique and massive mixture of more than 104 bacteria, archaea, and viruses3. The composition of microbiota in the gut differs from the microbiota found in other areas of the body3. Furthermore, different sections of an individual’s GI tract contain different microbiota compositions2. Many factors influence gut microbiota composition such as diet, health, environment, and birth delivery method2,7,8. The gut microbiota offers many essential functions and benefits to humans such as:- Digesting and fermenting complex carbohydrates1,2
- Aiding the immune system in defending against harmful microbes1,2
- Producing helpful metabolites and vitamins1,2
- Maintaining mucosal barrier function2
Gut Microbiota and Gut-Liver Crosstalk
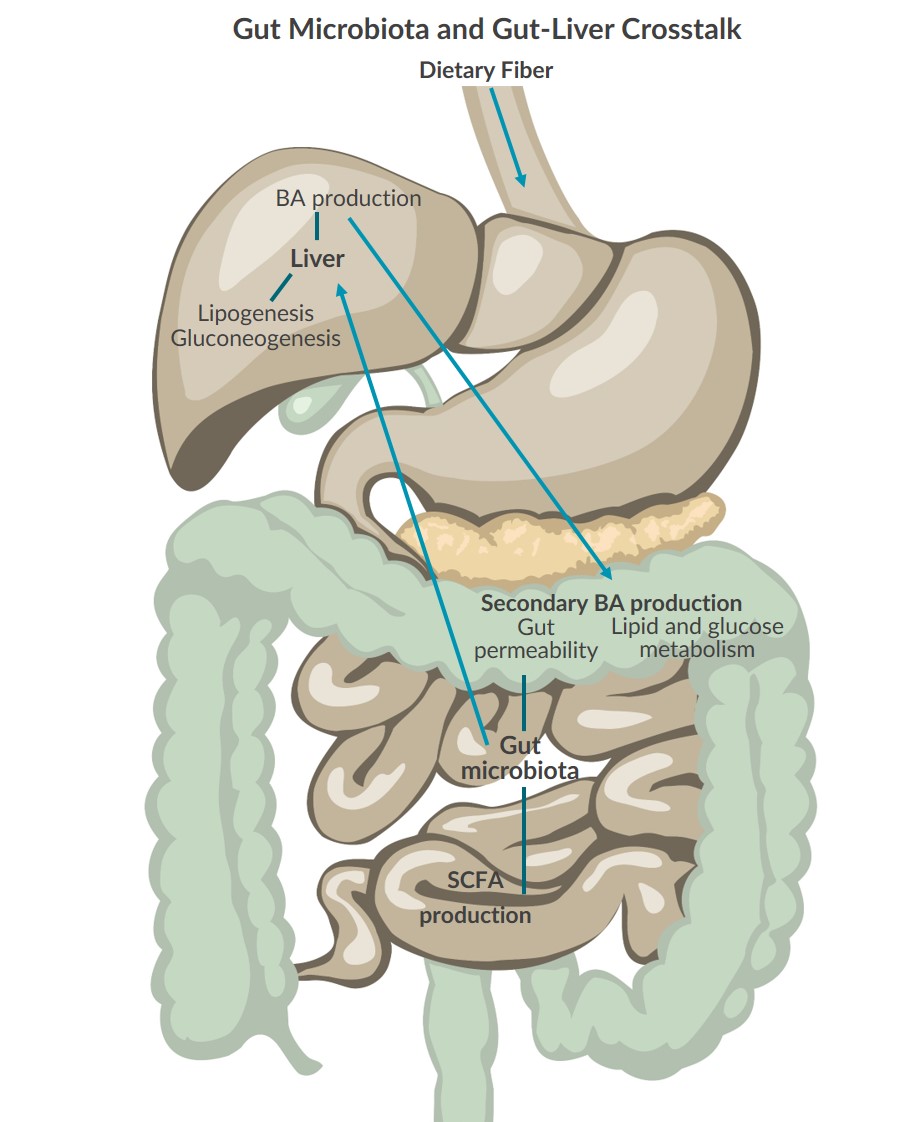
Healthy gut microbiota is now considered vital for proper metabolic function and regulation1,2. Research demonstrates that crosstalk between the gut microbiota and liver uses different types of biomolecules to communicate back and forth, supporting effective energy metabolism2,8.Communication from the Gut Microbiota to the Liver
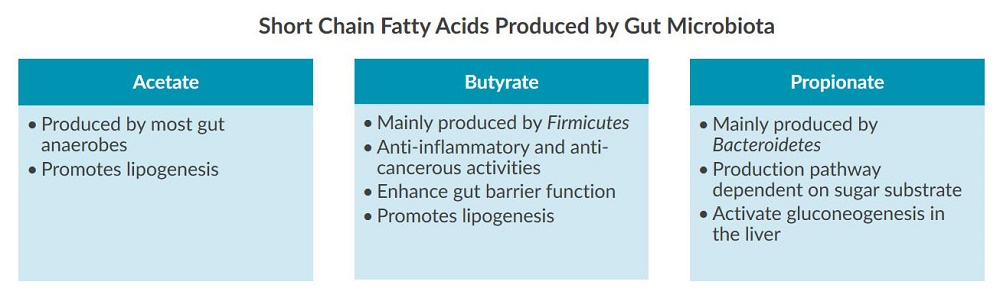
The gut microbiota talks to the liver through short chain fatty acids (SCFAs) produced during the digestion and fermentation of complex carbohydrates provided by dietary fiber2,8. SCFAs such as acetate, butyrate, and propionate are absorbed by GI epithelia cells and liver cells where they influence lipogenesis, gluconeogenesis, inflammation, and gut permeability2:
Communication from the Liver to the Gut Microbiota
In return, the liver produces bile acids (BAs) which interact with the gut microbiota in several ways2,7. BAs promote gut bacteria spore production, guide bacteria to the gut, and help gut microbiota recover from events such as antibiotic usage2. Furthermore, BAs are converted into secondary bile acids (or conjugated bile acids) by gut microbiota which are regulators of lipid and glucose metabolism, as well as gut permeability7,9. Additionally, research suggests that the gut microbiota regulates bile acid synthesis, diversity, and distribution7.
The Role of Gut Permeability in NAFLD
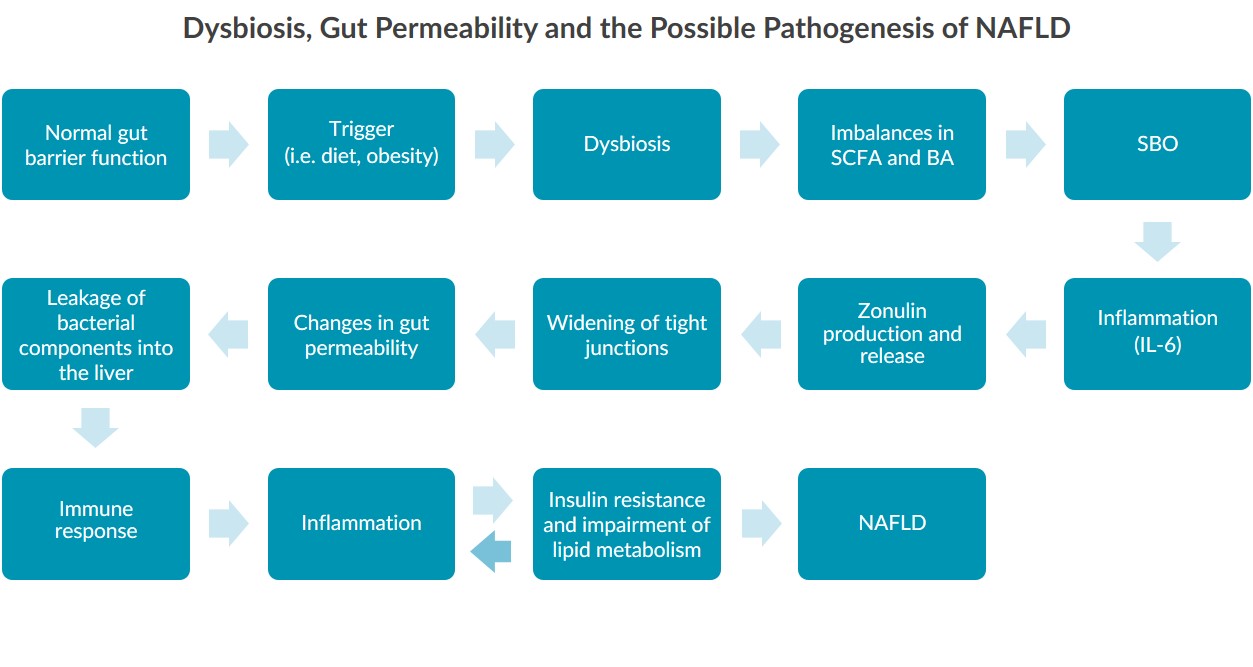
Research in mouse and human models indicates that alterations to the gut microbiota, referred to as dysbiosis, may be linked to the pathogenesis of NAFLD by affecting gut permeability3,5,6,7,8,9. Studies show that individuals with NAFLD have altered gut microbiota composition, including reductions in some species and increases in others3. Other studies reveal that individuals with NAFLD have impaired gut permeability3. Therefore, researchers are striving to uncover the complete mechanism linking dysbiosis to gut permeability and NAFLD.Zonulin in NAFLD
The interest in understanding the role of gut permeability in NAFLD has led some researchers to examine the GI protein zonulin. Zonulin can reversibly regulate gut permeability by modulating the size of tight junctions (TJs) between intestinal cells10,11,12. Therefore, researchers are highly interested in zonulin’s potential as a biomarker for NAFLD4,5,6,8 and have already found that:- Increased zonulin levels are associated with obesity and insulin resistance4,8
- Adults with NAFLD have higher serum zonulin levels than controls5
- Zonulin levels correlate with body mass index and IL-6 levels5
- Children with NAFLD have increased zonulin levels6
- Steatosis severity in children with NAFLD increases with zonulin levels6
 The gut microbiota is a unique collection of microorganisms vital for proper gut-liver crosstalk and maintaining homeostasis1,2. Biomolecules produced by the liver and microbiota aid in gut-liver crosstalk and support various metabolic functions2,7. Although the gut microbiota provides many benefits to humans, changes in its composition can lead to imbalances in SCFA and BAs, inflammation, and changes to gut permeability2,3,6,13. As such, studies show increased zonulin levels in NAFLD, which may indicate its involvement in the 4,5,6. More research is necessary, however, to fully understand the relationship between the gut microbiota and gut-liver crosstalk, as well as the role of gut permeability in NAFLD.
The gut microbiota is a unique collection of microorganisms vital for proper gut-liver crosstalk and maintaining homeostasis1,2. Biomolecules produced by the liver and microbiota aid in gut-liver crosstalk and support various metabolic functions2,7. Although the gut microbiota provides many benefits to humans, changes in its composition can lead to imbalances in SCFA and BAs, inflammation, and changes to gut permeability2,3,6,13. As such, studies show increased zonulin levels in NAFLD, which may indicate its involvement in the 4,5,6. More research is necessary, however, to fully understand the relationship between the gut microbiota and gut-liver crosstalk, as well as the role of gut permeability in NAFLD.
References
- Herrema et al. (2016). Emerging role of intestinal microbiota and microbial metabolites in metabolic control. Diabetologia. 2017 Apr;60(4):613-617. doi: 10.1007/s00125-016-4192-0.
- Thursby & Juge. (2017). Introduction to the human gut microbiota. Biochemical Journal. 2017 Jun 1;474(11):1823–1836. PMCID: PMC5433529.
- Tilg et al. (2016). How does the microbiome affect liver disease? Clinical Liver Disease. 8:123–126. doi:10.1002/cld.586.
- Moren-Navarrete et al. (2012). Circulating zonulin, a marker of intestinal permeability is increased in association with obesity-associated insulin resistance. PLoS ONE. 7(5):e37160. org/10.1371/journal.pone.0037160.
- Hendy et al. (2017). Evaluation of circulating zonulin as a potential marker in the pathogenesis of nonalcoholic fatty liver disease. APMIS. 2017 Jul;125(7):607-613. PMID: 28430371.
- Pacifico et al. (2014). Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol. 2014 Dec 7;20(45):17107–17114. PMCID: PMC4258579.
- Bashiardes et al. (2016). Non-alcoholic fatty liver and the gut microbiota. Mol Metab. 2016 Sep;5(9):782–794. PMCID: PMC5004228.
- Vajro et al. (2013). Microbiota and gut-liver axis: A mini review on their influences on obesity and obesity related liver diseases. J Pediatr Gastroenterol Nutr. 2013 May;56(5):461–468. PMCID: PMC3637398.
- Dia & Wang. (2015). Role of gut barrier function in the pathogenesis of nonalcholic fatty liver disease. Gastroenterol Res Pract. 2015;2015:287348. PMID: 25945084.
- Fasano et al. (2000). Zonulin, a newly discovered modulatory of intestinal permeability, its expression in coeliac disease. Lancet. 2000 Apr 29;355(9214):1518-9. PMID: 10801176.
- Fasano. (2008). Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: Living life on the edge of the wall. Am J Pathol. 2008 Nov;73(5):1243–1252. PMCID: PMC2570116.
- Tripathi et al. (2009). Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci. USA. 2009. PMID: 19805376.
- Miele et al. (2013). Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19(29):5314-24. PMID: 23432669.