[Infographic] The Benefits of Therapeutic Drug Monitoring for IBD
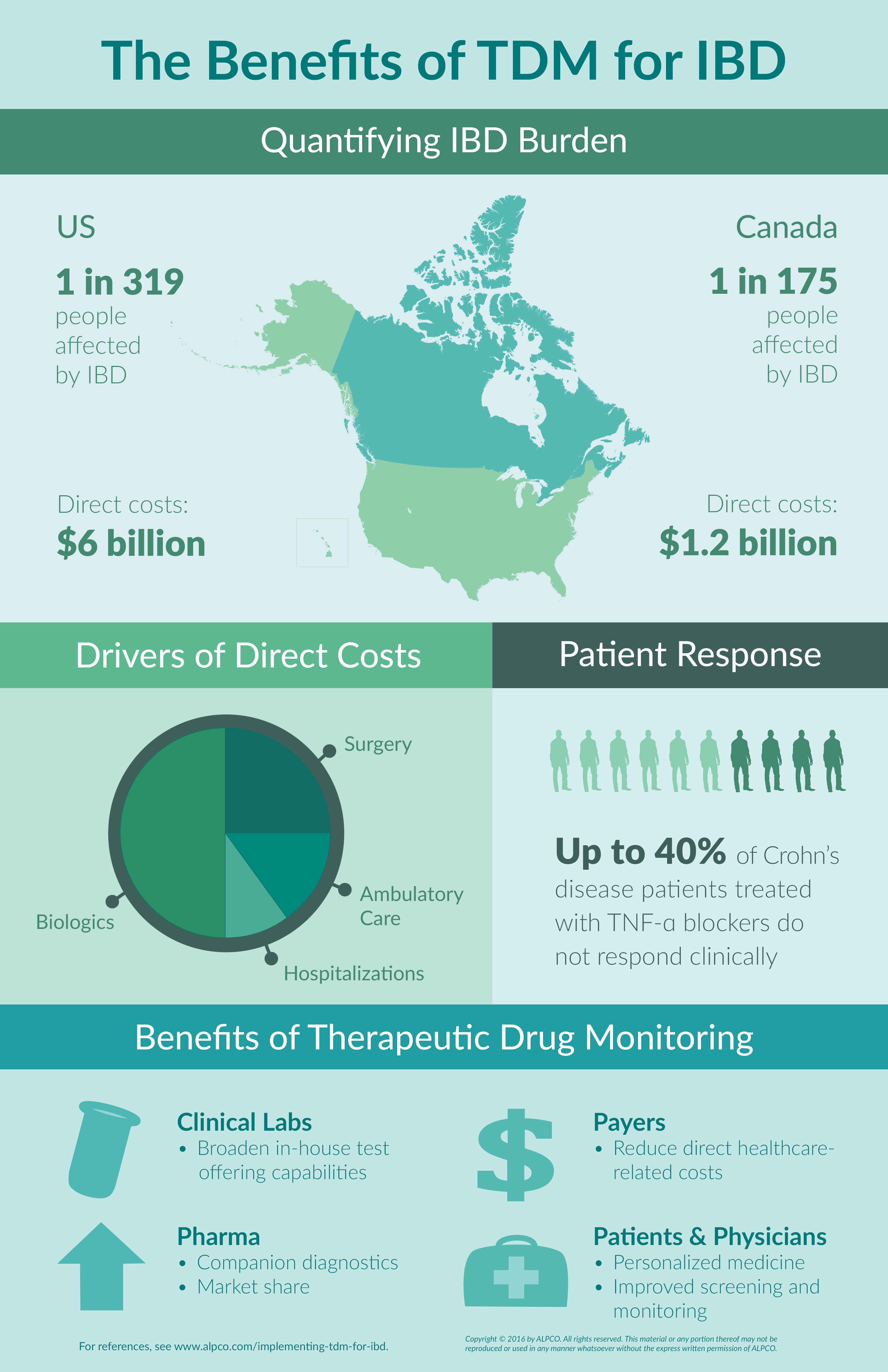
More than 1 million people in the US and 200,000 people in Canada are currently living with inflammatory bowel disease (IBD). This number is projected to grow exponentially over the next 10 years1,2. As the prevalence of IBD increases, so do direct healthcare costs. Between hospitalizations, surgery, ambulatory care and biologic therapy, Americans spend $6 billion annually on direct healthcare while Canadians spend $1.2 billion.2,3 Therapeutic drug monitoring (TDM), however, offers the opportunity to minimize this burden. Furthermore, the benefits of therapeutic drug monitoring for IBD are not only felt by patients and their physicians, but also stakeholders involved in treatment and testing including clinical reference labs, healthcare providers, and pharmaceutical companies.
The Cost of Having IBD
The major driver of high costs for patients in the US and Canada is related to biologics used to treat IBD.4 When taking into consideration the different subgroups within IBD that do not respond (40% of Crohn’s disease), do not achieve remission after 6 months (50-70%), or do not maintain response (30%), it is evident that a significant amount of money is being spent on treatments that are not optimized for the patient.5
The Benefits of Therapeutic Drug Monitoring for IBD
Therapeutic drug monitoring (TDM) for inflammatory bowel disease offers the opportunity to improve these figures and enhance the efficacy of biologics by allowing for dose and/or therapeutic changes. When implementing TDM for IBD, drug levels and antibodies against the drugs can be measured, providing insight into resistance and response. In turn, the benefits of therapeutic drug monitoring for IBD are felt by different subgroups involved in treatment and testing such as clinical reference labs, healthcare providers, and pharmaceutical companies, as well as patients and their physicians. To clinical reference labs, TDM extends the opportunity to broaden testing portfolios. To patients and their physicians, implementing TDM for IBD offers improved screening and monitoring processes, while also providing a path to personalized medicine. Healthcare providers are then relieved of a portion of direct healthcare costs, as TDM ensures patients are prescribed the right biologic at the right dose. Finally, pharmaceutical companies have the option to present their drug alongside a companion diagnostic, which may result in better retention of market share and improved patient outcomes. All of this demonstrates how the benefits of therapeutic drug monitoring for IBD can be felt by patients and stakeholders within the field alike.
References
- Kappelman et al. (2013). Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig. Dis. Sci. 58, 519–525.
- Rocchi et al. (2012). Inflammatory bowel disease: a Canadian burden of illness review. Can. J. Gastroenterol, 26, 811–817.
- Kappelman et al. (2008). Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology, 135, 1907–1913.
Marchetti & Liberato. (2014). Biological therapies in Crohn’s disease: are they cost-effective? A critical appraisal of model-based analyses. Expert Rev. Pharmacoecon. Outcomes Res., 14, 815–824. - Nielsen et al. (2011). Use of biological molecules in the treatment of inflammatory bowel disease. J. Intern. Med., 270(1), 15-28.

![[Infographic] The Benefits of Therapeutic Drug Monitoring for IBD](https://www.alpco.com/media/amasty/blog/cache/b/e/424/211/benefits-of-therapeutic-drug-monitoring-for-ibd.png)