Role of IGF-1 in the Growth Hormone/IGF Axis
The Growth Hormone/IGF axis consists of IGF-1, IGF-2 and several high and low affinity IGF Binding Proteins (IGFBP). The whole system is tightly regulated by a feedback loop involving Growth Hormone (GH) secreted by the pituitary, and GH production and secretion controlled by Growth Hormone Releasing Hormone (GHRH) at the hypothalamus.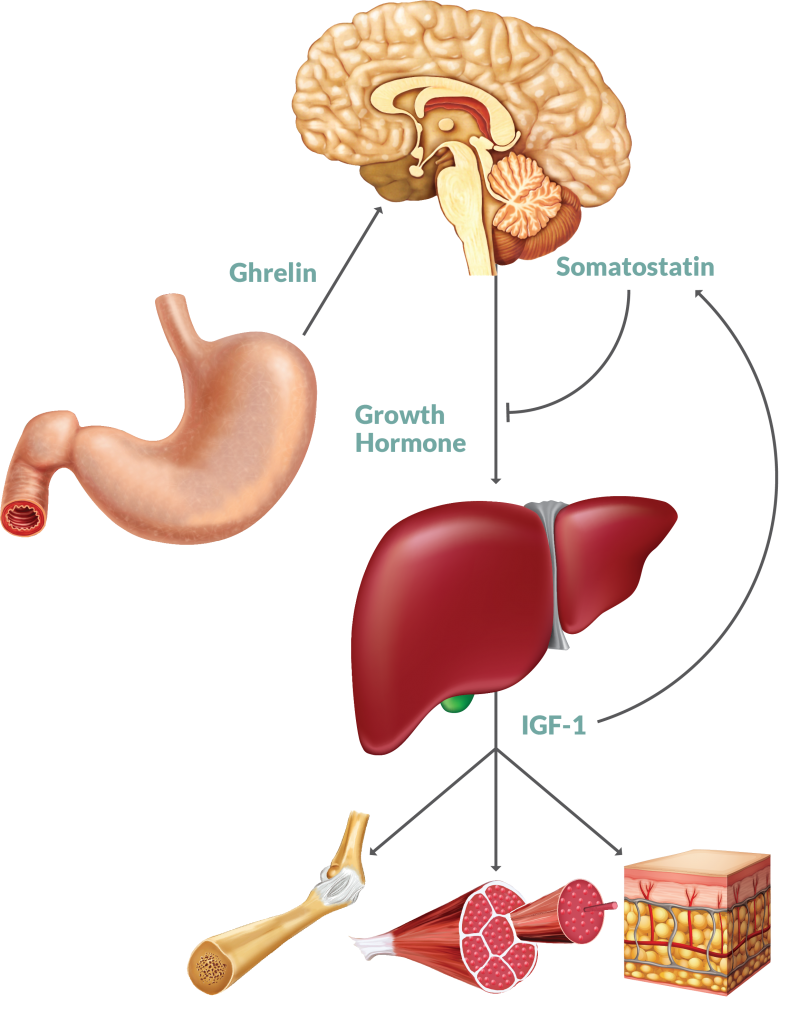
Insulin-like Growth Factors & Binding Proteins
Insulin-like growth factors (IGF) are involved in the proliferation and function of nearly every cell, tissue and organ in the human body. The IGF protein family consists of signaling proteins (IGF-1, IGF-2), cell membrane receptor proteins (IGF-1R, IGF-2R) with tyrosine kinase activity and a series of IGF binding proteins (IGFBP1 - IGFBP6). The IGFs’ mechanism of action is mediated through IGF/IGFR binding, kinase activation and the initiation of intracellular signaling via the AKT signaling pathway.1,2 The IGFBPs may modulate the action of IGFs in several different ways:
-
Inhibitory Model - IGFBPs prevent IGF/receptor association
-
Enhancing Model - IGFBPs transport IGFs and facilitate binding with IGF receptors
-
IGF Receptor-Independent Model - Product of direct IGFBPs/IGFBP receptor interaction
-
Proteolytic Cleavage of IGFBP - Cleaved into lower affinity fragments which increases freely circulating, bioavailable IGF
IGF-1
IGF-1 (Somatomedin C) is one of several growth factors necessary for normal human development. IGF-1 is synthesized mainly by the liver but also locally in many tissues. In contrast to many other peptide hormones, IGFs are avidly bound to specific IGFBPs, which significantly increases the half-life of IGF-1 in circulation.The seven classes of IGFBPs which are known at present1,3,4 either bind IGF-1 and IGF-2 with similar affinities or show a preference for IGF-2.5,6 The predominant IGFBP is IGFBP-3, which binds not only IGF-1 but also the Acid-labile Subunit (ALS). Thus, most of the IGF-1 in circulation is bound within this ternary complex.
Study Significance of IGF-1
A deregulation or imbalance in the IGF system could have implications in a number of different disorders including perturbations with normal growth, cancer and diabetes. As such, the IGF protein family continues to be a viable target for new therapeutic agents.
Growth and Development
In newborns, basal IGF-1 levels are low, but as humans develop, the GH feedback loop and the regulation of IGF-1 become more pronounced.7 Circulating IGF-1 stimulates growth in all cells, from skeletal muscle and bone to organ tissue and vessel linings.2 Circulating levels of IGF-1 vary greatly depending on a number of intrinsic and extrinsic factors, including age, gender, genetics, nutritional status, physical activity and stress.7 Due to the tightly controlled relationship between IGF-1 and GH, a deficiency or excess in either hormone can result in physiological errors in metabolism such as dwarfism, pituitary gigantism and acromegaly.2,3 Dwarfism can result from a mutation in the hepatic GH receptor which yields insensitivity to secreted GH. As a consequence, IGF-1 is synthesized and secreted at suboptimal levels. With outright GH deficiency, the pituitary does not produce enough GH, which negatively impacts IGF-1 levels.2,3 Pituitary gigantism arises from excessive GH and IGF-1 prior to growth plate fusion. Should hormone excess present after epiphyseal plate fusion, the condition is referred to as acromegaly. Both disorders are extremely rare and symptoms can be intermittent, often confounded by coexisting conditions. The common therapeutic approach for these disorders is aimed at regulating circulating GH and IGF-1 levels using recombinant hormone therapy,8 but ongoing in vitro and in vivo studies are employing newer methods of treatment. To date, treatments have the greatest impact when started prior to puberty.
Cancer
IGF-1 is a significant signaling molecule with regards to cancer cell transformation and proliferation, and IGF-1/IGF-1R binding and activation is the key step in downstream events including mitogenesis and apoptosis inhibition.1 When IGF-1 binds to IGF-1R, the tyrosine kinase activates the phosphoinositide 3-kinase (PI3K)-AKT pathway and many integral proteins involved in cellular metabolism, apoptosis, cell adhesion, and angiogenesis are regulated within this pathway. Numerous in vitro and in vivo studies have been performed that focused on the modulation of total and free IGF-1 levels and relative impact on tumor growth, invasiveness and response to therapeutics.6,9 These treatments range from siRNA knockdowns in mammalian tissue culture to targeted antibodies (biologics) specific for the IGF-1R.1,3,9 Inhibition of IGF-1R activation via small molecule inhibitors or a biologics could be a large step towards identifying viable cancer therapies.
Diabetes
IGF-1 is highly homologous with insulin and can readily bind to the insulin receptor (IR). However, the IGF-1R exhibits a significantly higher affinity for IGF-1 binding than for the IR. Due to this relationship, scientists have theorized that IGF-1 can be used to treat diabetes, especially if started prior to puberty.2,3 Preliminary studies have demonstrated positive results in this approach, yet the mechanism by which IGF-1 treatments might correct for insulin resistance clinically remains unclear.2
