November 9, 2021
Insulin Resistance: Root Causes, Detection, and Prevention
The Diabetes Pandemic
According to the U.S. Centers for Disease Control (CDC) and the World Health Organization, more than 34 million Americans and 422 million people worldwide have diabetes.1,2 90 to 95% of people with diabetes have type 2.1 The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) estimates that more than 1 in 3 U.S. adults (~84 million) have prediabetes or insulin resistance. Worldwide, this could translate to more than 850 million individuals.3 Diabetes is a worldwide pandemic and cases are steadily on the rise. Thankfully, in most cases, insulin resistance and type 2 diabetes are both preventable and treatable.What is Insulin Resistance?
Insulin resistance describes a state where more insulin is required to obtain the biological effects achieved by a lower amount of insulin in the normal state. Insulin resistance in tissues including the liver, skeletal muscle, and adipose tissue is an early step in the progression towards type 2 diabetes.4 Insulin resistance often involves an increase in lipid storage in muscle and/or liver tissue which leads to an impairment in insulin signaling and energy homeostasis, as well as increases in plasma levels of glucose and free fatty acids. If left unchecked, insulin resistance often leads to the development of metabolic syndrome, type 2 diabetes, liver disease, and cardiac disease.5 Though aging, hereditary factors, and other, less-studied phenomenon have been implicated in the etiology of resistance, over-nutrition is the most common cause of insulin resistance.5,6 If the rate of food supply continually exceeds demand, cells eventually transition to lipid storage, ultimately causing an increase in intramyocellular fat content in muscle and liver cells.5 An abnormal increase in lipid content within muscle cells, hepatocytes, adipocytes, and in the circulation leads to inflammation and impaired insulin function through a number of potential pathways.Muscle Insulin Resistance
In a healthy individual, increased insulin production and binding to muscle cells following a mixed meal stimulates glucose uptake and metabolism through a chain of intracellular events.5,7 [caption id="attachment_8799" align="alignleft" width="344"]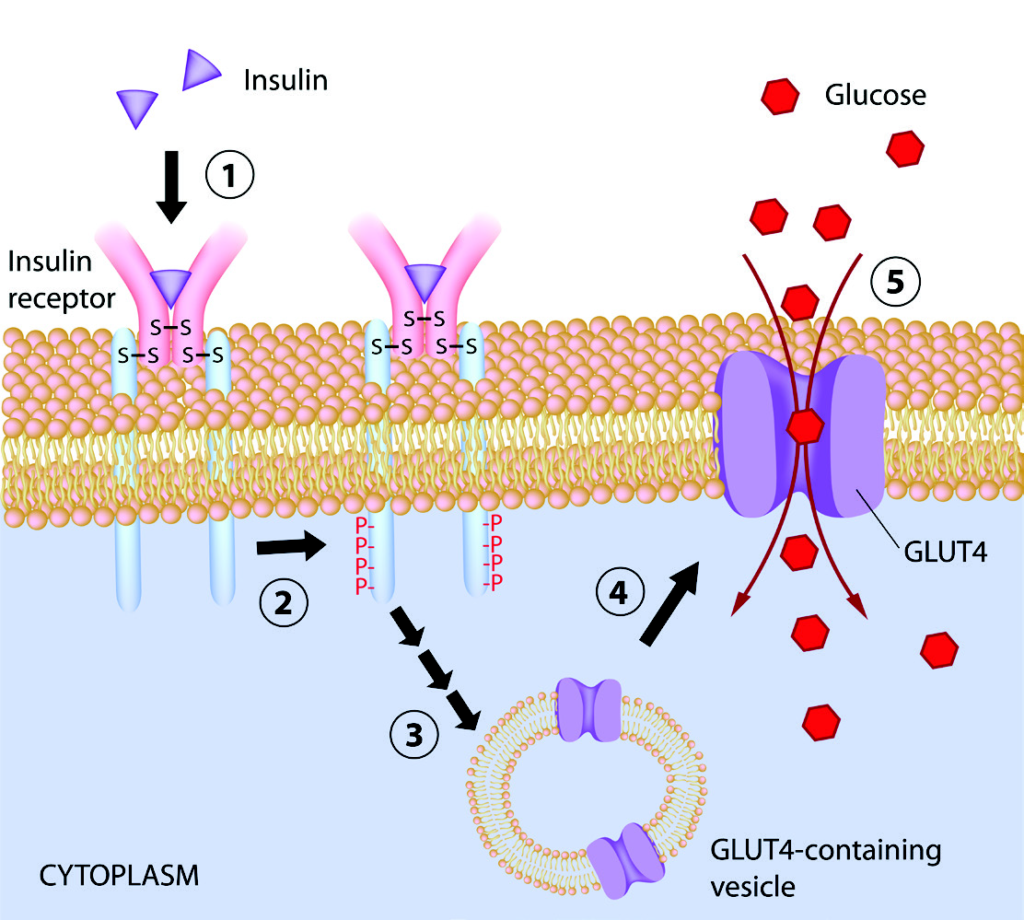 Figure 1. Insulin function in muscle cell.[/caption]
Step 1. Insulin binds to insulin receptor
Step 2. Insulin binding stimulates phosphorylation of insulin receptor substrate-1 (IRS-1)
Step 3. IRS-1 activates PI 3-kinase
Step 4. Activated PI 3-kinase activates glucose transporter 4 (GLUT4)
Step 5. GLUT4 facilitates uptake of glucose into the cell
Step 6. Glucose is converted to energy in the mitochondria
Figure 1. Insulin function in muscle cell.[/caption]
Step 1. Insulin binds to insulin receptor
Step 2. Insulin binding stimulates phosphorylation of insulin receptor substrate-1 (IRS-1)
Step 3. IRS-1 activates PI 3-kinase
Step 4. Activated PI 3-kinase activates glucose transporter 4 (GLUT4)
Step 5. GLUT4 facilitates uptake of glucose into the cell
Step 6. Glucose is converted to energy in the mitochondria
Research Review: Understanding Insulin Resistance
Download
Looking for our full list of Diabetes and Obesity testing solutions?
View Product Listing
When more lipid is stored and processed in muscle cells, lipid metabolites activate protein kinase C which then blocks the phosphorylation of IRS-1 in step 2 of the insulin-signaling pathway. In essence, excess accumulation of intracellular lipids in muscle cells prevents proper insulin signaling and the uptake of glucose into the muscle cells, a state known as insulin resistance.7
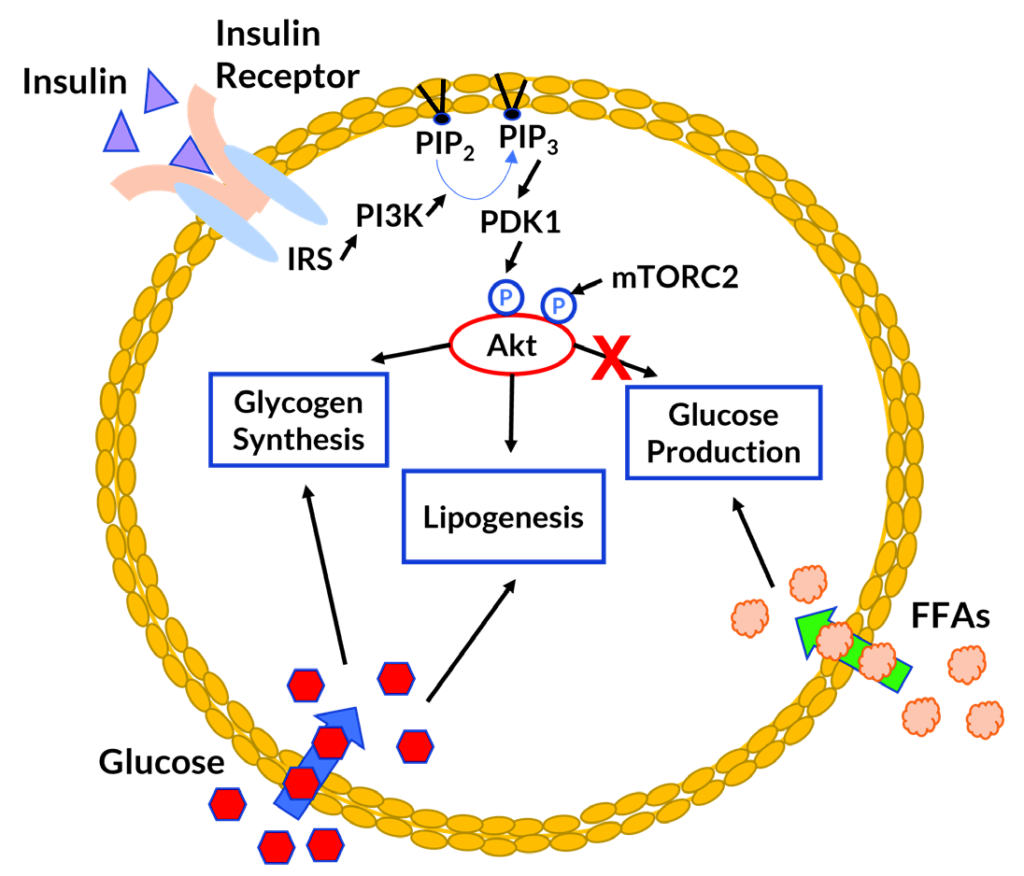 Figure 2. Insulin function and energy homeostasis in hepatocyte (liver cell).[/caption]
Insulin plays a key role in the regulation of glucose and lipid metabolism in the liver. In a properly functioning liver, insulin binding increases glucose uptake and signals the cells to stop glucose production. Cells convert glucose to glycogen for storage. As levels of glycogen rise, cells begin to store excess energy in the form of lipids.8
In muscle insulin resistance, glucose transport into the cell is impaired. Some of the glucose not taken up by muscle cells is diverted to the liver.9 Increased circulation and uptake of glucose promotes glycogen and lipid production in the liver. In hepatic insulin resistance, insulin binding fails to stop glucose production and glucose accumulation promotes lipid synthesis.8
Figure 2. Insulin function and energy homeostasis in hepatocyte (liver cell).[/caption]
Insulin plays a key role in the regulation of glucose and lipid metabolism in the liver. In a properly functioning liver, insulin binding increases glucose uptake and signals the cells to stop glucose production. Cells convert glucose to glycogen for storage. As levels of glycogen rise, cells begin to store excess energy in the form of lipids.8
In muscle insulin resistance, glucose transport into the cell is impaired. Some of the glucose not taken up by muscle cells is diverted to the liver.9 Increased circulation and uptake of glucose promotes glycogen and lipid production in the liver. In hepatic insulin resistance, insulin binding fails to stop glucose production and glucose accumulation promotes lipid synthesis.8
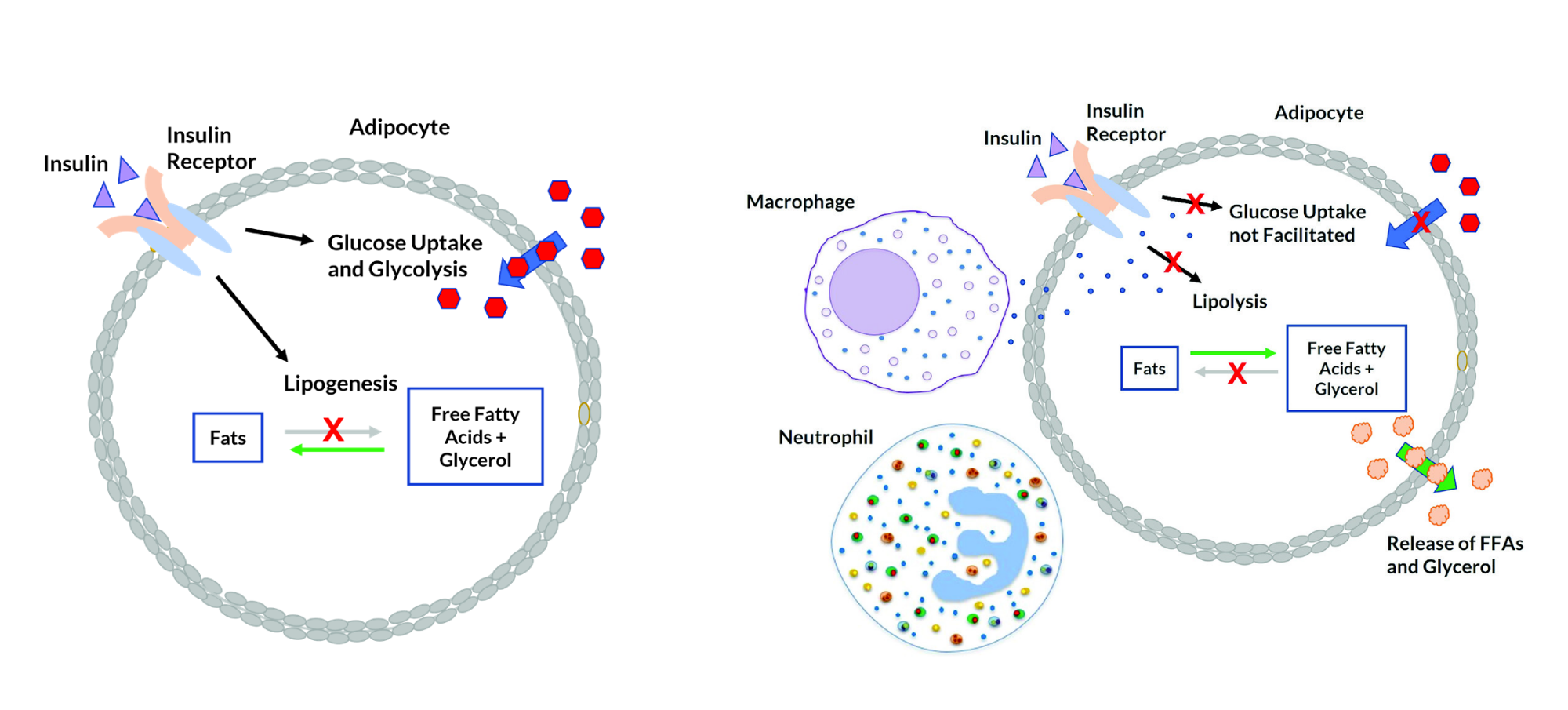
Figure 3. Normal insulin function in adipocyte (fat cell). Figure 4. Insulin resistance in adipocyte (fat cell). [/caption]
Hepatic (Liver) Insulin Resistance
[caption id="attachment_8800" align="alignright" width="313"] Figure 2. Insulin function and energy homeostasis in hepatocyte (liver cell).[/caption]
Insulin plays a key role in the regulation of glucose and lipid metabolism in the liver. In a properly functioning liver, insulin binding increases glucose uptake and signals the cells to stop glucose production. Cells convert glucose to glycogen for storage. As levels of glycogen rise, cells begin to store excess energy in the form of lipids.8
In muscle insulin resistance, glucose transport into the cell is impaired. Some of the glucose not taken up by muscle cells is diverted to the liver.9 Increased circulation and uptake of glucose promotes glycogen and lipid production in the liver. In hepatic insulin resistance, insulin binding fails to stop glucose production and glucose accumulation promotes lipid synthesis.8
Figure 2. Insulin function and energy homeostasis in hepatocyte (liver cell).[/caption]
Insulin plays a key role in the regulation of glucose and lipid metabolism in the liver. In a properly functioning liver, insulin binding increases glucose uptake and signals the cells to stop glucose production. Cells convert glucose to glycogen for storage. As levels of glycogen rise, cells begin to store excess energy in the form of lipids.8
In muscle insulin resistance, glucose transport into the cell is impaired. Some of the glucose not taken up by muscle cells is diverted to the liver.9 Increased circulation and uptake of glucose promotes glycogen and lipid production in the liver. In hepatic insulin resistance, insulin binding fails to stop glucose production and glucose accumulation promotes lipid synthesis.8
White adipose tissue can exert systemic effects on energy and lipid homeostasis through the release of free fatty acids (FFAs) and glycerol into the bloodstream.9 Increased circulation and binding of free fatty acids in a state of insulin resistance further promotes glucose, glycogen, and lipid production in the liver.8 Excess storage of lipids in liver cells is a hallmark of hepatic insulin resistance and non-alcoholic fatty liver disease (NAFLD).9
While muscle insulin resistance often precedes hepatic insulin resistance, diets that are high in fat have been shown to cause hepatic insulin resistance without muscle insulin resistance by blocking insulin-stimulated AKT phosphorylation in the liver. High-fructose diets promote hepatic insulin resistance by suppressing glucokinase activity and glycogen synthesis.9Adipose Tissue and Inflammation in Insulin Resistance
In a healthy individual, insulin binding to adipocytes stimulates glucose uptake, suppresses lipolysis, and promotes lipid synthesis. Insulin binding tells the fat cells to store energy as fat. Lipid synthesis and storage in the fat cells reduces plasma levels of free fatty acids (FFAs) and glycerol.8 In obese individuals, there is an increase in macrophages and neutrophils in the adipose tissue. Cytokine production by the macrophages can interfere with insulin receptor signaling in fat cells, causing increased lipolysis and the release of free fatty acids and glycerol into the bloodstream.6 Obesity-related hypothalamic inflammation and proinflammatory cytokine production can lead to suppression of leptin signaling and failure to feel the sensation of being full, known as leptin resistance. Inflammation in the central nervous system can further contribute to signaling changes and systemic insulin resistance.6 [caption id="attachment_8804" align="aligncenter" width="739"]
Figure 3. Normal insulin function in adipocyte (fat cell). Figure 4. Insulin resistance in adipocyte (fat cell). [/caption]
The Role of Aging and Heredity
Studies suggest that a decrease in the activity of mitochondrial enzymes, due to oxidative damage to mitochondria over time, may be associated with normal aging. A decrease in mitochondrial activity (and processing of glucose for energy production) in muscle cells of up to 35% may lead to an increase in lipids. This increase in lipids and intramyocellular lipid metabolites, a major inhibitor of insulin signaling and glucose transport, causes insulin resistance in muscle.5-7,9 In healthy, young, lean offspring of parents with type 2 diabetes, overall mitochondrial activity in muscle cells can be reduced by almost 40%. This appears to be related to an overall reduction in mitochondrial density and biogenesis that is genetic. Again, reduced mitochondrial activity and glucose usage leads to an increase in lipids. The increase in intramyocellular lipid metabolites leads to muscle insulin resistance.5-7,9Detecting Insulin Resistance
Not surprisingly, obesity, particularly high visceral or abdominal fat, is the greatest risk factor for insulin resistance and type 2 diabetes. Insulin resistance or prediabetes is often associated with high levels of fasting glucose (due to malfunctions in glucose processing) and triglycerides (due to increased lipid production, lipolysis, and release of free fatty acids).3 In several studies, hepatic diacylglycerol content has shown to be a strong indicator of hepatic insulin resistance. Some studies have also shown a positive correlation between insulin resistance and leptin and resistin levels,9 and a negative correlation between insulin resistance and adiponectin.4,9,10 Fibroblast growth factor 21 (FGF21), lipocalin-2 and other biomarkers are being investigated in the pursuit of enhanced screening for insulin resistance.4 Dysbiosis, or alterations in the gut microbiota, have been associated with obesity, insulin resistance, and diabetes. Some potential mechanisms for involvement of the microbiota include inflammation induced by low-grade endotoxemia, increased energy harvest from food, altered fatty acid metabolism, and variations in gut-derived peptide secretion.4 There are documented cases where fecal transplants from lean to obese individuals have led to reductions in body fat.6 A greater understanding of the microbiome in relation to healthy and disease states is required, but there is future potential for microbiome profiling in the prevention and diagnosis of insulin resistance and type 2 diabetes.4,6Preventing and Treating Insulin Resistance
There are a variety of therapeutic agents under investigation that work to decrease lipid storage in muscle and liver cells, increase insulin sensitivity, reduce lipolysis, or reduce inflammation. Metformin, a popular diabetes drug, functions by inhibiting glucose production in the liver. Therapy utilizing leptin, the satiety hormone that causes us to feel full, has been shown to reduce caloric intake, increase weight loss, and improve insulin function.9 A better understanding of the link between diet, specific micro-organisms within the microbiome, and energy metabolism may yield new treatments (including probiotics) that may assist patients in managing cravings, achieving weight loss goals, reversing insulin resistance, and avoiding related disorders.6 Specific therapies geared toward mitochondrial function may someday assist individuals with insulin resistance that is not caused by over-nutrition. In obese individuals, weight loss and exercise have been shown to completely reverse insulin resistance and type 2 diabetes.5,6,9 Aerobic exercise has been shown to activate muscle glucose transport by an insulin- independent mechanism, enabling the normal processing of dietary glucose into muscle glycogen and energy.9 Despite advancements in therapeutics, a healthy diet and exercise, with a reduction in overall caloric intake and reduced consumption of high-fat, high-fructose, and highly processed foods remain the most effective weapons in the fight against insulin resistance and diabetes. References- CDC (2020). Type 2 Diabetes. https://www.cdc.gov/diabetes/basics/type2.html. Accessed 21Oct21.
- WHO (2021). Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 21Oct21.
- NIDDK (2018). Insulin Resistance & Prediabetes. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/prediabetes-insulinresistance. Accessed 21Oct21.
- Park SE (2015). Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Critical Reviews in Clinical Laboratory Sciences. 52(4).
- Abdul-Ghani MA and DeFronzo RA (2010). Pathogenesis of Insulin Resistance in Skeletal Muscle. Journal of Biomedicine and Biotechnology. Volume 2010, Article ID 476279.
- Johnson, AMF and Olefsky JM (2013). The origins and drivers of insulin resistance. Cell. 152: 673-684.
- Petersen KF, MD and Shulman GI, MD, PhD (2006). Etiology of Insulin Resistance. 119(5 Suppl 1): S10–S16.
- Santoleri D and Titchenell PM (2019). Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 7:447–456.
- Samuel VT and Schulman GL. (2016). The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126(1):12-22.
- Sakou FE-L, Reine, et al (2021). Inflammatory biomarkers and prediction of insulin resistance in Congolese adults. Heliyon. 7:e061392.

