Differentiating IBD from IBS with Fecal Calprotectin Testing
In an effort to improve patient care, an increasing number of gastroenterologists are using fecal calprotectin levels as a first step in the diagnosis of IBD versus IBS. ALPCO tests enable clinicians to accurately assess patients via calprotectin and quickly start patients on the right treatment path.
What is Calprotectin?
Calprotectin is a calcium- and zinc-binding protein produced and released by neutrophils recruited to sites of infection and inflammation.1,2 It is highly resistant to pancreatic proteases and bacterial degradation in the intestine, making it very stable in stool samples. The amount of calprotectin present in stool is directly proportional to the number of neutrophils present and the degree of inflammation in the intestines.2
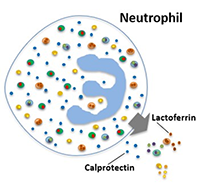

Differentiating IBD from IBS with Calprotectin
Inflammatory bowel disease (IBD) is an inflammatory disease that causes chronic swelling and damage within the digestive tract. Irritable bowel syndrome (IBS) is a non-inflammatory intestinal disorder. While the symptoms of both can be very similar, the recommended treatments for IBD and IBS are quite different.3
Though colonoscopy remains the definitive test for a positive IBD diagnosis, the American College of Gastroenterology Task Force determined in 2010 that “routine colonic imaging is not recommended in patients younger than 50 years of age with typical IBS symptoms and no alarm features.”4
Fecal calprotectin levels are being used to noninvasively stratify patients presenting symptoms of IBD and IBS before recommending a colonoscopy. Clinical studies and FDA submissions for ALPCO calprotectin tests have validated that calprotectin levels above the conventional clinical cut-off of 50 μg/g in stool are indicative of mucosal inflammation associated with IBD and results can be used to effectively differentiate IBD from IBS.5-10
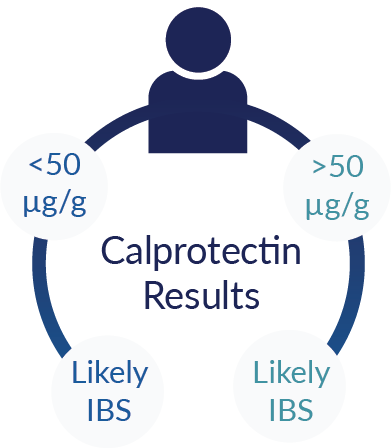

Prioritizing Colonoscopy for Suspected IBD Patients
More than 19 million colonoscopies were performed in the United States in 2019.11 With screening colonoscopies for colorectal cancer on the rise and limited availability of colonoscopy in some geographic areas, wait times can be a concern for some patients. When clinicians at Eastern Health in Victoria, Australia used low calprotectin values to omit patients from colonoscopy and very high levels to schedule priority colonoscopies, the wait time for patients with high levels of calprotectin was reduced by 50%. This demonstrates the utility of calprotectin screening to stratify suspected IBD patients and improve efficiency of care.14
Generate Fast, Accurate Calprotectin Test Results with ALPCO
ALPCO offers a growing range of FDA cleared tests for the accurate, reproducible determination of fecal calprotectin. ALPCO tests combine the clinical sensitivity and specificity required to effectively aid in the differentiation of IBD from IBS with workflow efficiency and reliability for prompt reporting of quantitative calprotectin results.
ALPCO Calprotectin Immunoturbidimetric Assay
The Calprotectin Immunoturbidimetric Assay is an in vitro diagnostic particle-enhanced immunoassay for the quantitative measurement of fecal calprotectin. The assay is compatible with most clinical chemistry analyzers, enabling automated analysis without a capital purchase.


Clinically Accurate
- Clinical sensitivity of 90.5% - few false negatives
- Clinical specificity of 93.4% - few false positives


Reliable
- AMR of 11 -10,000 µg/g* - broad range of reportable quantitative results
- Inter-lot CV of only 4.1% - repeatable performance


Fast
- Testing of >400 samples / hour** - high throughput
- STAT results in 10 minutes** - fast turn-around


User Friendly
- Compatible with most clinical chemistry analyzers - minimal training and hands-on time
- Universal stool extraction device - single preparation with no manual weighing


Cost Effective
- Only 2 non-reportables per day - minimal cost for low and high volume testing
- Cost savings vs. send-out with revenue potential and CAGR of 6%
*With automatic 1:10 dilution
**Based on the Beckman AU680 analyzer
Put ALPCO’s Calprotectin Immunoturbidimetric Assay to the Test.
Your Calprotectin Testing, Elevated.
Contact ALPCO to discuss workflow efficiencies and cost savings associated with a switch to the ALPCO calprotectin immunoturbidimetric assay.
Take Control of Your Calprotectin Testing.
See for yourself why labs are switching to ALPCO’s new FDA-cleared Calprotectin Immunoturbidimetric Assay.
One free kit per laboratory. Void where prohibited.
Not Ready to Automate?
ALPCO offers an FDA-cleared and Health Canada licensed Calprotectin Chemiluminescence ELISA.
References
- McMahon CW, Chhabrta R (2018). The role of fecal calprotectin in investigating digestive disorders, J. Lab. Precis. Med., 3:19.
- Bjarnason I (2017). The use of fecal calprotectin in inflammatory bowel disease, Gastroenterol. Hepatol., 13(1), 53-56.
- Premier Health [Online] (2017). Bowel Basics: Is it IBD or IBS? Available at: https://www.premierhealth.com/your-health/articles/women-wisdom-wellness-/bowel-basics-is-it-ibs-or-ibd [Accessed 20 Feb 2020].
- Chey WD, et al (2010). The Yield of Colonoscopy in Patients With Non-Constipated Irritable Bowel Syndrome: Results From a Prospective, Controlled US Trial, Am J Gastroenterol., 105:4, 859-865.
- An Y, et al (2019). Fecal calprotectin testing for identifying patients with organic gastrointestinal disease: systematic review and meta-analysis, MJA, 211(10), 461-467.
- Manz M, et al (2012). Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study, BMC Gastroenterology, 12:5.
- Tibble, et al (2000). A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000 Oct; 47(4): 506–513. PMID: 10986210.
- Gisbert and McNicholl (2009). Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009 Jan;41(1):56-66. PMID: 18602356.
- Waugh, et al (2013). Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technology Assessment, No. 17.55. Southampton (UK): NIHR Journals Library; 2013 Nov. PMID: 24286461.
- Walsham and Sherwood (2016). Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016; 9: 21–29. PMID: 26869808.
- iData Research (2018). An Astounding 19 Million Colonoscopies are Performed Annually in The United States. Idataresearch.com.
- Hubers J, et al (2020). Trends in Wait Time for Colorectal Cancer Screening and Diagnosis 2013-2016, Clinical and Translational Gastroenterology.
- Canadian Partnership Against Cancer [Online], 2017, Screening reports show many Canadians still waiting too long for final diagnosis of breast and colorectal cancer. Available at: https://www.partnershipagainstcancer.ca/news-events/news/article/screening-reports-show-many-canadians-still-waiting-long-final-diagnosis-breast-colorectal-cancer/ [Accessed 20 Feb 2020].
- Motaganahalli S, et al (2019). Faecal calprotectin delivers on convenience, cost reduction and clinical decision-making in inflammatory bowel disease: a real-world cohort study, Internal Medicine Journal, 49, 94-100.







