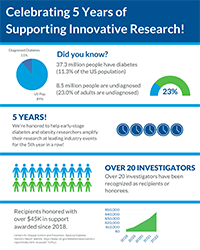
ALPCO strives to provide scientists and healthcare professionals with robust tools for their research and clinical diagnostic endeavors. We understand that championing extraordinary discoveries requires extraordinary efforts, and support. We are proud to continue our Diabetes Research Travel Grant program which has awarded more than $45K in support to over 20 investigators.
Working towards advancements in life science research and improved clinical outcomes
ALPCO’s Diabetes Research Travel Grant
About the Diabetes Research Travel Grant
To further promote progressive diabetes and obesity research, ALPCO sponsors a travel grant award program.
This year, ALPCO is celebrating five years of supporting innovative research through our Diabetes Research Travel Grant!


2024 Recipient: Hannah Foster, University of Wisconsin - Madison
Incretin mimetics (e.g., semaglutide and tirzepatide) are widely recognized diabetes therapeutics making national headlines. These drugs are designed to target the GLP1 receptor, which is expressed on pancreatic β-cells. But how does GLP1 receptor signaling boost insulin secretion? Hannah has discovered that GLP1 receptor signaling activates the glycolytic enzyme pyruvate kinase, which increases cAMP production beyond what the GLP1 receptor is capable of alone. This finding could be the basis of the next generation of biased GLP1 receptor agonists that more efficiently raise insulin secretion.
The ALPCO Diabetes Travel Grant will support Dr. Foster in presenting her research at relevant meetings.
Please join us in celebrating Hannah and her achievement!
Apply for ALPCO’s Diabetes Research Travel Grant
Eligibility
All applicants must be currently enrolled graduate students, postdoctoral fellows, or early stage investigators* in North America and must be primarily responsible for the research described. All successful applicants must commit to submitting/presenting their results in the form of a scientific poster or oral presentation at a relevant diabetes conference within one year from award receipt. Conferences include: ADA, MIC, BIIC, ABC, and Keystone Symposia. Please inquire if you would like to propose an alternative conference. An applicant is eligible to receive one award per year.
Criteria
Competitive applications will describe active research which clearly and concisely demonstrates direct relevance to diabetes and obesity research, treatment and/or future therapies.
Award Features
The grant consists of:
- $2,500 product credit for applicable ALPCO assays** (to be purchased within six months of award)
- Up to $2,500 to present abstract/findings (to be used within one year of award), including:
- Travel fees (airfare and lodging only)***
- Conference registration fees***
Application Materials
Submissions must include the following:
- Diabetes Research Travel Grant application
- Research description
- Advisor/mentor letter
- Current resume/CV
Research Description
The description should include your abstract and a clear explanation of the connection between the research and its relevance to improving future diabetes and obesity treatment or therapies. The length of the proposal should not exceed two pages (including figures and references).
Advisor/Mentor Letter
We require that the applicant’s scientific advisor/mentor also send a letter confirming enrollment or postdoctoral status. The letter must detail the applicant’s contribution to the research, distinguishing it from the efforts of other supporting team members, and establishing the significance of the contribution to its relevant scientific discipline.
Submission
Please email all application materials to: [email protected].
Past Diabetes Research Travel Grant Recipients


2023 Recipient: Mallory Brayer, The George Washington University
We’re pleased to announce that ALPCO’s Diabetes Research Travel Grant has been awarded to Mallory Brayer, PhD Student, at the George Washington University.
Mallory’s research explores the mechanisms by which low-frequency therapeutic ultrasound (TUS) stimulates insulin release from rodent and human pancreatic beta-cells. In her current sono-genetic studies, she is also investigating the effect of TUS stimulation on gene expression in the pancreas with a focus on secretory proteins and channels regulating insulin secretion from human islets.
This emerging concept of TUS-mediated mechano-sensation and signaling in pancreatic islets may open novel non-pharmacological and non-invasive strategies for treatments of type-2 diabetes mellitus. The ALPCO Diabetes Travel Grant will support Ms. Brayer in presenting her research at relevant meetings.


Winter 2022 Recipient: Anoop Arunagiri, University of Michigan
We’re pleased to announce that ALPCO’s Diabetes Research Travel Grant has been awarded to Anoop Arunagiri, PhD, a Research Investigator at the University of Michigan.
Dr. Arunagiri’s research is centered on studying proinsulin misfolding in pancreatic beta cells, and delineating the events and factors contributing to misfolding in the context of diabetes.
Proinsulin misfolding has been implicated as a primary cause of pancreatic beta cell failure, either in the presence of INS gene mutations that trigger misfolding, or in the development of type 2 diabetes (T2D). The origin of proinsulin misfolding in T2D however is not well understood. Dr. Arunagiri utilized several anti-proinsulin antibodies, applied in Western blotting protocols, to help define proinsulin misfolding and its relationship to diabetes progression. Working together with Dr. Peter Arvan (University of Michigan), he has determined the immunoblotting conditions necessary to detect as well as quantify the abundance of non-native misfolded proinsulin in both human pancreatic islets and in animal models. Dr. Arunagiri’s studies using T2D mice revealed the prevalence of aberrant intermolecular disulfide-linked complexes of proinsulin as one of the important events in the natural history of progression of beta cell failure in type 2 diabetes (Arunagiri A et al., eLife (2019) doi:10.7554/eLife.44532). This research has energized the whole field with the realization that fixing misfolding is a key to helping prevent beta cell failure leading to diabetes.


Summer 2021 Recipient: Mollie Huber, University of Florida
Mollie’s novel research utilizes live human and mouse pancreatic tissue slices to study the genuine islet microenvironment in autoimmune Type 1 Diabetes. Her work seeks to address knowledge gaps about how the early stages of diabetes autoimmunity affect islet cell function prior to beta cell loss.
Mollie is developing methods to use live human and mouse pancreas slices to understand the pathogenic process that occurs during diabetes. In addition to evaluating islet function, she has also developed methods for identifying T cells as well as the antigens these immune cells recognize in the slices from donors with diabetes.
The Phelps and Mathews labs were the first to visualize live and functional human insulitis and Mollie has since made additional insulitis observations. The methods she developed will allow her to measure islet function in the context of insulitis (the hallmark pathological lesion of Type 1 diabetes). A full detailing of islet function/dysfunction when insulitis is present will allow for the development of therapies to promote beta cell function as well as those that prevent islet dysfunction.
Mollie’s studies are poised to provide translational information regarding how human islets lose function during the early asymptomatic phase of Type 1 diabetes as well as the mechanisms that drive the dysfunction. The travel award will allow Mollie to present her work at a relevant diabetes conference.


Winter 2021 Recipient: Xi Wang, Cornell University
Xi’s research focuses on the development of a transplantable encapsulation device for the safe delivery of functional, insulin-producing beta cells.
She developed a tubular, Nanofiber Integrated Cell Encapsulation (NICE) device with nanofibrous skin and a hydrogel core. This device design successfully prevented cell penetration or escape and protected stem cell-derived β (SC-β) cells from immune attack, supporting cell function and insulin production. The encapsulated SC-β cells reversed diabetes immediately following transplantation and maintained proper function for up to 120 days in immunodeficient mice and up to two months in immunocompetent mice. As a proof of concept, the scalability and retrievability of the device containing human SC-β cells was demonstrated in a canine model through a minimally invasive laparoscopic procedure.
Xi’s work suggests the potential for a new therapeutic approach in the treatment of type 1 diabetes.


Summer 2020 Recipient: Erica Cai, Harvard Medical School
Dr. Cai’s research focuses on understanding the mechanism of autoimmunity pertaining to beta cell destruction and type 1 diabetes (T1D). She initiated a project that uses a genome-wide CRISPR screen approach in a mouse model for T1D to search for genetic modifications that can protect transplanted beta cells against autoimmune destruction.
This innovative method identified renalase, which has been previously associated with T1D risk. By inhibiting renalase, she demonstrated that transplanted beta cells in mice can be protected, leading to disease inhibition or delay. Her research not only indicates that renalase is a modifier of beta cell vulnerability, but that the enzyme is a potential therapeutic target to avert beta cell loss in T1D.1 Dr. Cai’s research has also identified FDA-approved pargyline as a drug that can mimic the effects of renalase inhibition.
Dr. Cai’s work at the Joslin Diabetes Center showcases that a genome-wide CRISPR screen approach is a powerful method for novel target discovery, opening new avenues for researching diabetes therapies.

Winter 2020 Recipient: Marlena Holter, Cornell University
Ms. Holter’s novel research focuses on GLP-1 regulated glucose regulation following vertical sleeve gastrectomy surgery to define the mechanisms underlying type 2 diabetes remission and metabolic improvement following bariatric surgery. She is utilizing a mouse model to investigate the connection between β-cell GLP-1R signaling and α-cell proglucagon processing to identify targets that can switch α-cells from producing glucagon to GLP-1.
Her work at Cornell represents a substantial contribution to the definition of a new paradigm in pancreatic islet biology and the identification of novel mechanisms to target α-cells for the prevention and treatment of type 2 diabetes. Ms. Holter’s research embodies the mission of ALPCO’s grant and helps build the foundation for improving the quality of life for people living with diabetes.
With the award, Ms. Holter will be able to present her findings at a relevant diabetes conference sometime between 2020-2021.


Summer 2019 Recipient: Dan Tremmel, University of Wisconsin
Mr. Tremmel’s novel research focuses on the use of human pancreas-derived extracellular matrix (ECM) to improve the 3D in vitro culture of stem cell derived beta cells for transplantation. He has developed a decellularization protocol to isolate the ECM from human pancreatic tissue which can then be formed into a hydrogel and used to study how the pancreas-specific ECM influences beta cell maturation and function.
His doctorate work at the University of Wisconsin has the potential to support the survival and functions of islets during islet and stem cell-derived beta cell transplantation and is aligned with ALPCO’s mission to advance research and improve the quality of life for those living with type 1 and type 2 diabetes. Mr. Tremmel presented his poster Reconstructing the In Vivo Environment to Elucidate the Role of the Islet Niche in January 2020 at the Islet Biology: From Gene to Cell to Micro-Organ Keystone Symposia in Santa Fe, NM.


Winter 2019 Recipient: Lisa Volpatti, Massachusetts Institute of Technology
Ms. Volpatti’s work with microgel encapsulated nanoparticles for glucose-responsive insulin delivery demonstrates excellent innovation and potential for new therapies to enhance the lives of people living with type 1 and advanced type 2 diabetes. The development of glucose-responsive materials for self-regulated insulin delivery marks a significant advancement in the precise regulation of blood sugar levels for the treatment of diabetes.
Ms. Volpatti presented her research Microgels Encapsulating Glucose-responsive Nanoparticles for Self-Regulated Insulin Delivery in July 2019 at the Controlled Release Society’s annual meeting in Valencia, Spain. Learn more about Ms. Volpatti’s ongoing research at lisarvolpatti.com.

Summer 2018 Recipient: Stephen M. Grote, Saint Louis University School of Medicine
Mr. Grote’s work with the novel peptide neuronostatin shows great promise to the diabetes research community. His research suggests that neuronostatin has an effect on glucagon synthesis and release, while also proposing that the peptide may have therapeutic potential in the treatment and prevention of hypoglycemia and alpha cell dysfunction in diabetes.
Mr. Grote presented his research A New Player in Intra-islet Communication: The Hyperglycemic Effects of Neuronostatin through Increased Glucagon Production and Reduced Insulin Release in April 2019 at Experimental Biology in Orlando, FL.
Frequently Asked Questions
What does a complete application include?
Submissions must include the following:
- Diabetes Research Travel Grant application
- Research description: The description should include your abstract and a clear explanation of the connection between the research and its relevance to improving future diabetes and obesity treatment or therapies. The length of the proposal cannot exceed two pages (including figures and references).
- Advisor/mentor letter: We require that the applicant’s scientific advisor/mentor also send a letter confirming enrollment or postdoctoral status. The letter must detail the applicant’s contribution to the research, distinguishing it from the efforts of other supporting team members, and establishing the significance of the contribution to its relevant scientific discipline.
- Current resume/CV
How do I submit my application?
You can submit your application and submission materials to: [email protected].
For additional information, please contact:
Megan Castilla
Product Manager, Life Science Research Segment
603-681-2577
When will I find out if I have been awarded a grant?
The award winners will be announced 90 days after the application deadline.
If I am awarded a grant, which shows can I choose to present at next year?
Conferences include: ADA, MIC, BIIC, ABC, and Keystone Symposia. Please inquire if you would like to propose an alternative conference.
If I receive a grant, how do I submit my receipts for reimbursement?
Please submit all receipts to [email protected].
ALPCO reserves the right to change or discontinue the grant program at any time.
*Early stage investigators: less than 10 years from terminal degree.
**Applies to kits with a catalog number starting with “80-”.
***Award winners will be responsible for arranging their own travel, accommodations, and conference registration. Receipts will be required to receive reimbursement.