BTK Inhibitors as Cancer Drug Treatments
July 26, 2016: Over the past several years, the drug arsenal to combat B cell cancers has gained a mighty weapon: Bruton’s tyrosine kinase (BTK) inhibitors. The origins of using BTK inhibitors as cancer drug treatments actually began with researching drug treatments for an autoimmune disease. Two decades later, the first BTK inhibitor to achieve FDA approval was Imbruvica (Ibrutinib) and it has proven to be an effective treatment for multiple B cell cancers that rely on a fully functional B cell receptor (BCR) pathway and BTK signaling in order to progress1,2.
The BCR Pathway and BTK Signaling
Although Imbruvica (Ibrutinib) has been successful in treating several B cell cancers, the BTK inhibitor was originally designed as a potential rheumatoid arthritis (RA) drug at Celera Genomics3. BTK inhibitors were selected due to their ability to interfere with cell signaling cascades and prevent key phosphorylations in B cells, therefore preventing or reducing inflammation4. Since RA is a chronic inflammatory autoimmune disorder with pathogenesis based in B cell dysfunction5, BTK seemed to be a natural target to explore. The team at Celera Genomics successfully developed “irreversible” BTK inhibitors that, when introduced to the BCR pathway, tightly clung to the kinase via covalent bonds, preventing subsequent phosphorylations3. One compound showed promising results by inhibiting RA development in mice3.
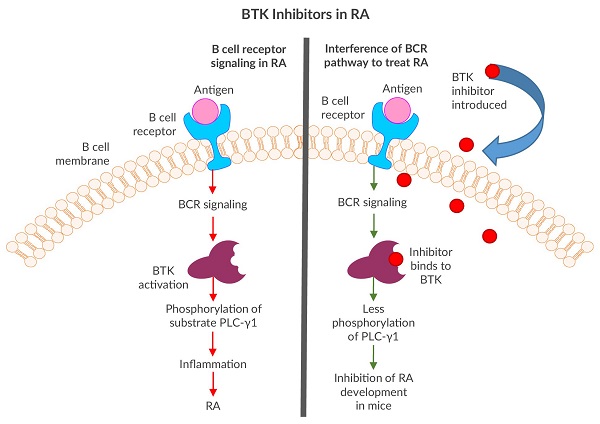
From Arthritis to B Cell Cancer Drug Development
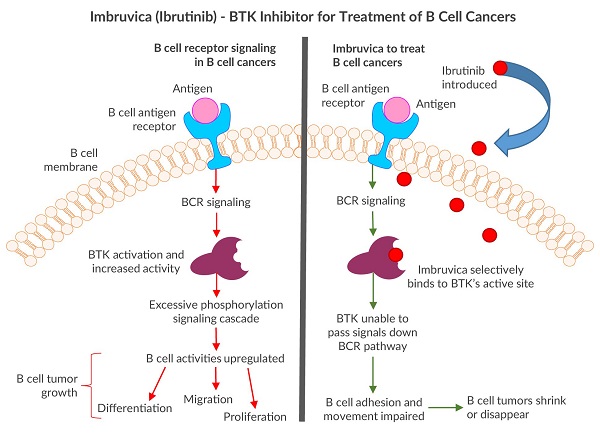
When Pharmacyclics licensed several compounds from Celera Genomics in 2006, they chose to further develop one BTK inhibitor, now known as Imbruvica (Ibrutinib)6. Pharmacyclics took the drug in a different direction and focused on BTK’s ability to regulate B cell activities such as differentiation, migration, proliferation, and apoptosis to use against B cell cancers7.
BTK Inhibitors as Cancer Drug Treatments
The first clinical trial for Imbruvica was a great success. It demonstrated how the BTK inhibitor managed to shrink or eliminate some types of B cell tumors by disrupting the BCR pathway and lowering the cancerous B cells’ ability to move around and adhere7. Additionally, only mild adverse side effects were observed and key organs such as the liver and kidney were not harmed. During the trial, the drug proved to be the most effective in fighting against mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma7. This clinical trial led to the FDA approving Imbruvica for treating MCL in 20138. The following year, the drug was also approved to treat another B cell cancer, chronic lymphocytic leukaemia (CLL)9. Imbruvica has also proven to be an effective treatment for Waldenström’s macroglobulinemia (WM) and was given FDA approval in 201510. Utilizing BTK inhibitors as cancer drug treatments has come a long way, although there is still a need for more development. Imbruvica is the only FDA approved BTK inhibitor to treat B cell cancers. Pharmacyclics’ pipeline currently includes seven clinical trials using the drug to target additional B cell cancers such as diffuse large B cell lymphoma (DLBCL) and marginal zone lymphoma (MZL)11. With each FDA approval, Imbruvica helps more B cell cancer patients win their battles.
References
- Pharmacyclics. (2015). Our Science: Bruton’s Tyrosine Kinase (BTK) Inhibitors. Pharmacyclics.com.
- Janssen. (2014). B-cell signaling. Janssen Pharmaceuticals. bcellmalignancy.eu.
- Pan et al. (2007). Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem, 2(1), 58-61. PMID: 17154430.
- Petro et al. (2000). Bruton's tyrosine kinase is required for activation of Iκb kinase and nuclear factor κb in response to B cell receptor engagement. J. Exp. Med., 191(10), 1745–1754. PMID: 10811867.
- Silverman & Carson. (2003). Roles of B cells in rheumatoid arthritis. Arthritis Res. Ther., 5(Suppl 4), S1–S6. PMID: 15180890.
- Owensand & Krieger. (2016). Pharmacyclics’ ‘miracle cure’: A cancer drug saves a biotech company. San Jose Mercury News. Seattletimes.com.
- Advani et al. (2012). Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol., 31(1), 88-94. PMID: 23045577.
- Yao. (2013). FDA News Press Release: FDA approves Imbruvica for rare blood cancer. US Food and Drug Administration. FDA.gov.
- Yao. (2014). FDA News Press Release: FDA expands approved use of Imbruvica for chronic lymphocytic leukemia. US Food and Drug Administration. FDA.gov.
Goodin. (2015). FDA News Press Release: FDA expands approved use of Imbruvica for rare form of non-Hodgkin lymphoma. US Food and Drug Administration. FDA.gov.
Pharmacyclics. (2016). Pharmacyclics Pipeline. Pharmacyclics.om.
