March 15, 2016
Physiological Actions of Natriuretic Peptides
Introduction
Humans have at least 50 discovered hormones managing vital biological functions in the body. These hormones are produced by the body’s organs and glands. Natriuretic peptides (NPs) are structurally related hormones synthesized and released from the heart, including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). Due to some of the physiological actions of natriuretic peptides in the body, ANP and BNP are sometimes referred to as cardiac natriuretic hormones (CNHs)1. Research has shown that both ANP and BNP are aiding in cardiovascular, kidney, and diabetes research. Multiple forms of natriuretic peptides have been discovered, but some forms are easier to measure for research than others.Physiological Actions of Natriuretic Peptides
ANP and BNP are products of different genes found on human chromosome 1, the NPPA gene and the NPPB gene, respectively. Many physiological actions of natriuretic peptides have been discovered including the production of urine (diuresis), release of sodium in urine (natriuresis), and dilation of blood vessels (vasodilation)1-3. The heart’s atrium secretes ANP which helps reduce blood pressure1 and the ventricles secrete BNP, resulting in lower blood volume and pressure1. Together, these NPs contribute to the regulation of cardiovascular and renal homeostasis as well as blood pressure control by influencing the Renin-Angiotensin II-Aldosterone system (RAAS)1,4. The different physiological actions of natriuretic peptides have made the hormones key players in multiple areas of research such as cardiovascular disease, kidney disease, and diabetes.Natriuretic Peptides and Cardiovascular Disease
Over the years, ANP and BNP have proven to be valuable to cardiovascular research. Besides vasodilation, ANP and BNP can increase myocardial relaxation, and protect the cardiovascular system against hypertrophy and fibrosis1. Research has shown that levels of both NPs can increase in response to various cardiovascular diseases (CVDs)3. The physiological actions and changes in the concentrations of ANP and BNP explains why these NPs are considered novel biomarkers of CVD. Both ANP and BNP have been used to research different aspects of CVD including: ANP- Oxidative health
- Sepsis
- Cardiac hypertrophy
- Cardiac failure
- Cardiac failure
- Cardiac impairment
- Acute myocardial infarction
- Hypertension
Natriuretic Peptides and Kidney Disease
ANP and BNP also play physiological roles in the renal system such as increasing renal blood flow and glomerular filtration rate (GFR)1. Researchers have also used these NPs to investigate chronic kidney disease (CKD) and end stage renal disease (ESRD) due to their roles in renal homeostasis. Researchers have observed an increase in BNP concentrations in subjects with declining kidney function5, therefore it is possible BNP levels can help differentiate between stages of kidney disease6,7.Natriuretic Peptides and Diabetes
More recently, ANP and BNP have gained interest in diabetes and obesity research. Evidence suggests that ANP can influence glucose and fat metabolism by increasing adiponectin levels8 and can protect against the onset of diabetes9. Evidence has also shown BNP to be involved in both fatty acid metabolism and body weight regulation10. These findings suggest that BNP and ANP levels can help further the understanding of the connection between diabetes and CVD11. As research continues, there is increasing potential for NPs to be applied to drug development and treatments for CVD, kidney disease, and diabetes.Forms of Natriuretic Peptides
Several forms of natriuretic peptides exist and some are easier to measure than others. The prohormone forms of NPs undergo many cleavages to generate the biologically active forms of the hormone. The cleavages result in different fragments of various amino acid lengths. N-terminal (NT) prohormone fragments of natriuretic peptides are typically more stable, have longer half-lives, and circulate at higher concentrations compared to C-terminal biologically active hormone ends12,13. It has therefore been suggested that measuring NT prohormone fragments of ANP and BNP provide more accurate concentrations in samples2,3,14.NT-proANP vs. alpha-ANP
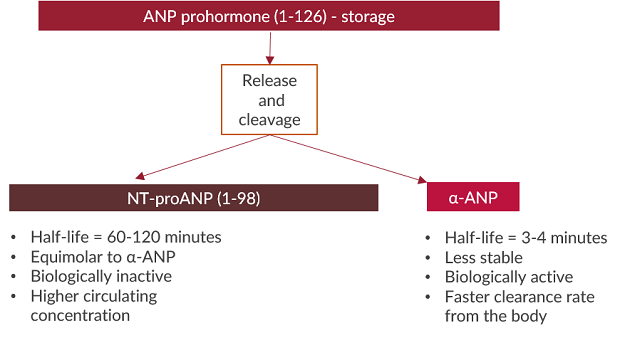
NT-proBNP vs. BNP
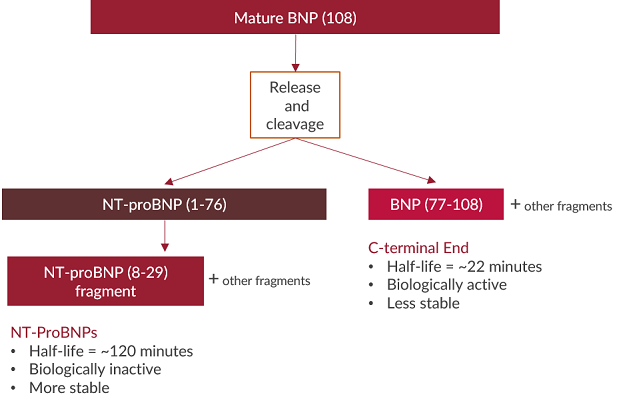
Conclusion
Over the years, it has been found that there are many physiological actions of natriuretic peptides in the body. ANP and BNP have proven to be vital to proper cardiovascular and kidney functions. As a result, measuring ANP and BNP has proven to be beneficial to cardiovascular, kidney, and diabetes research. Multiple forms of natriuretic peptides circulate throughout the body; however, research has shown that the NT pro-fragments of NPs are better for measuring concentrations.References
- Volpe. (2014). Natriuretic peptides and cardio-renal disease. International Journal of Cardiology, 176(2014), 630–639. doi:10.1016/j.ijcard.2014.08.032.
- Vesely. (2002). Atrial natriuretic peptide prohormone gene expression: hormones and diseases that upregulate its expression. IUBMB Life, 53, 153–9. PMID: 12102171.
- Clerico et al. (2011). Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol, 301, H12-H20. PMID: 21551272.
- Federico. (2010). Natriuretic peptide system and cardiovascular disease. Heart Views,11(1),10–15. PMCID: PMC2964706.
- Spanaus et al. (2007). B-Type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: The mild-to-moderate kidney disease study. Clinical Chemistry, 53(7), 1264-1272. doi: 10.1373/clinchem.2006.083170.
- Yasuda et al. (2012). Plasma B-type natriuretic peptide level predicts kidney prognosis in patients with predialysis chronic kidney disease. Nephrol. Dial. Transplant, 27(10), 3885-3891. doi: 10.1093/ndt/gfs365.
- Sakuma et al. (2010). Plasma B-type natriuretic peptide level and cardiovascular events in chronic kidney disease in a community-based population. Circ J, 74(4), 792-7. PMID: 20160392.
- Birkenfeld et al. (2012). Atrial natriuretic peptide and adiponectin interactions in man. PLoS ONE, 7(8), e43238. PMID: 22916229.
- Jujic et al. (2014). Atrial natriuretic peptide and type 2 diabetes development: Biomarker and genotype association study. PLOS, 9(2), e89201. PMID: 24586593.
- Moro & Smith. (2009). Natriuretic peptides: New players in energy homeostasis. Diabetes, 8, 27-26. PMCID: PMC2780882.
- Gruden et al. (2014). Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes Care, 37(11), 2899-2908. doi: 10.2337/dc14-0669.
- Yandle et al. (1986). Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci, 38, 1827–1833. PMID: 2939312.
- Arjamaa et al. (1996). Atrial plasma ANP and NH2-terminal proANP during right atrial pressure increase in humans. Acta Physiol Scand, 157(4), 481-5. PMID: 8869731.
- Iqbal et al. (2012, April 3). Is the new natriuretic peptide better than the old ones for diagnosing CHF. American College of Cardiology.
