March 12, 2018
Classes of Biologics for the Treatment of IBD
Monoclonal antibody (mAb) biologics have been used to treat inflammatory bowel disease (IBD) for several years. Various classes of biologics for the treatment of IBD exist and include cytokine blockers and integrin blockers. Within these classes are mAbs that target different proinflammatory proteins such as tumor necrosis factor-alpha (TNF-alpha), interleukin 12 and 23 (IL-12, IL-23), and integrins1.
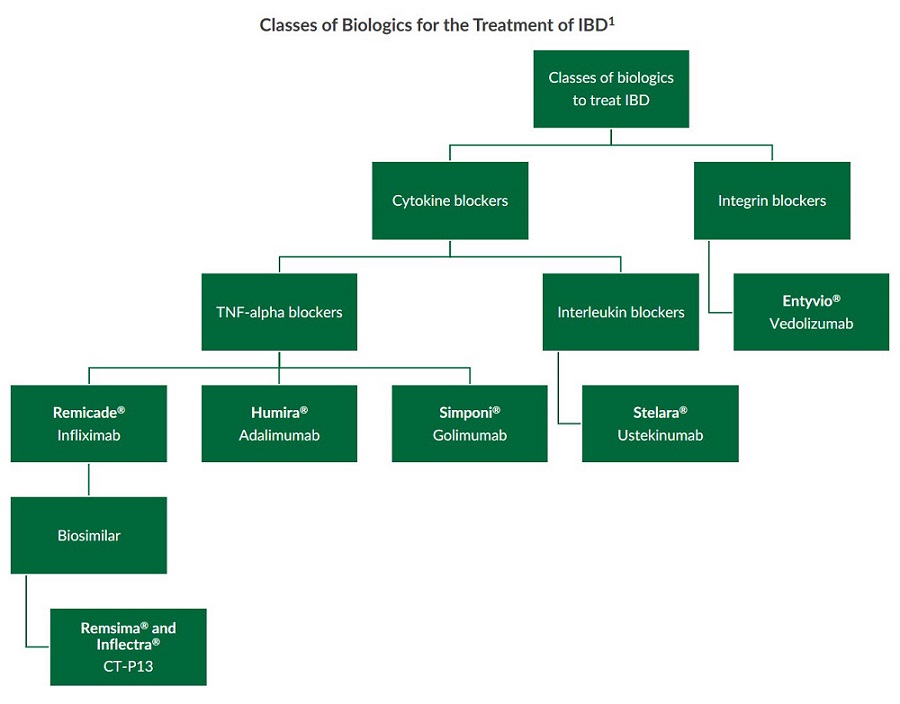
 Several classes of biologics for the treatment of IBD have been developed to suppress inflammation in the GI tract including TNF-alpha blockers, interleukin blockers, and integrin blockers1. These monoclonal antibody biologics successfully use different mechanisms to block the proinflammatory cell signaling of cytokines, interleukins, and proteins in the GI tract.
Several classes of biologics for the treatment of IBD have been developed to suppress inflammation in the GI tract including TNF-alpha blockers, interleukin blockers, and integrin blockers1. These monoclonal antibody biologics successfully use different mechanisms to block the proinflammatory cell signaling of cytokines, interleukins, and proteins in the GI tract.

TNF-alpha Blockers in IBD
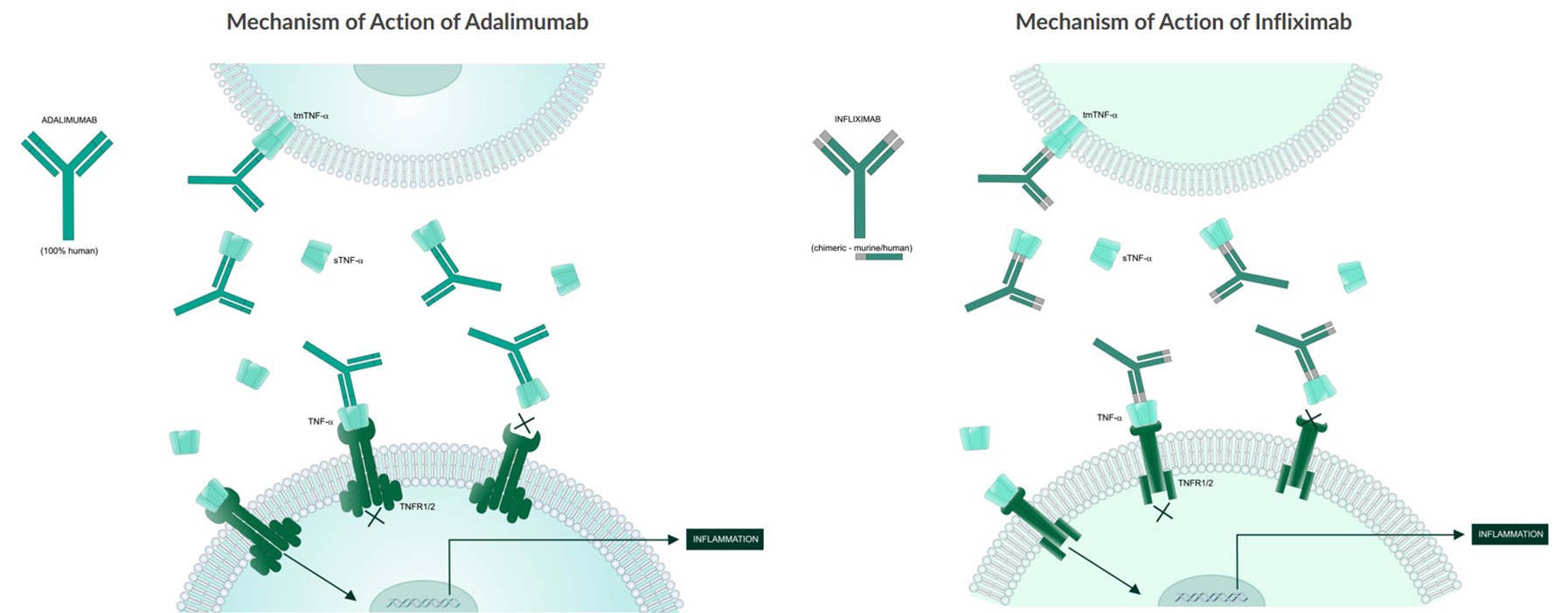
Both animal and human studies indicate that the proinflammatory cytokine TNF-alpha plays a pivotal role in the development and progression of IBD. Mice overexpressing TNF-alpha can develop a Crohn’s-like phenotype2. Furthermore, upon analysis of inflamed mucosa from patients with both ulcerative colitis (UC) and Crohn’s disease (CD), elevated levels of certain pro-inflammatory cytokines including TNF-alpha were found3. TNF-alpha blockers bind to TNF-alpha and prevent the cytokine from binding to its receptors. This inhibits the progression of an inflammatory cascade triggered by TNF-alpha in the gastrointestinal tract4. Remicade® (infliximab), Humira® (adalimumab), and Simponi® (golimumab) are examples of TNF-alpha blockers currently approved by the FDA for the treatment of IBD. Additionally, the infliximab biosimilar CT-P13 (Remsima® and Inflectra®) is now approved in several countries for the treatment of IBD.
Interleukin Blockers in IBD
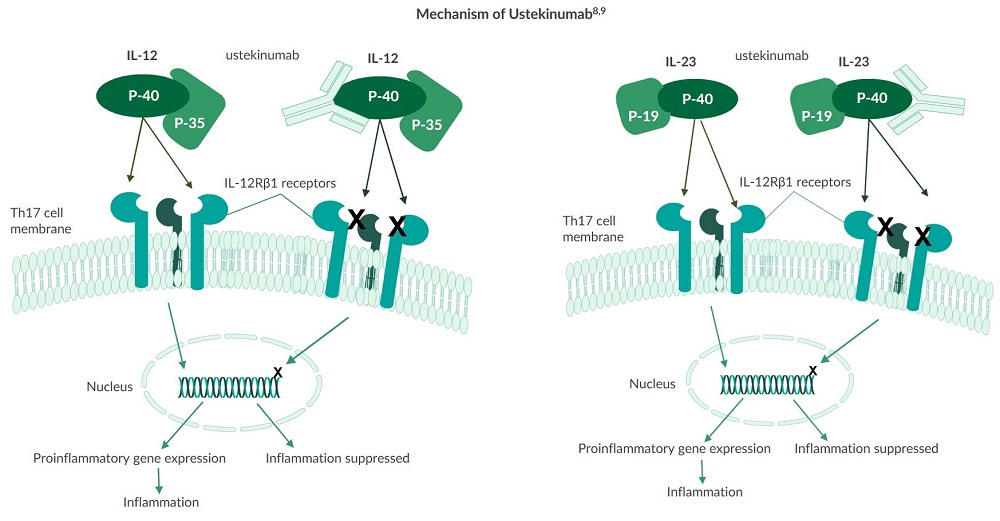
Humans generate a myriad of interleukins which are cytokines produced by white blood cells. Research demonstrates that both IL-12 and IL-23 signaling pathways are involved in the pathogenesis of IBD5. Excessive IL-12 is produced and secreted by white blood cells within the intestinal lamina, while gene variants for the IL-23 receptor have been strongly associated with the development of CD5. Stelara® (ustekinumab) is the first biologic, targeting both IL-12 and IL-23, to be approved by the FDA for the treatment of Crohn’s disease6,7. This interleukin blocker targets the p40 subunit found in both interleukins and prevents them from binding to IL-12Rβ1 receptors on T cells. As a result, fewer T1 and T17 helper cells are recruited and activated, reducing inflammation in the gut5.
Integrin Blockers in IBD
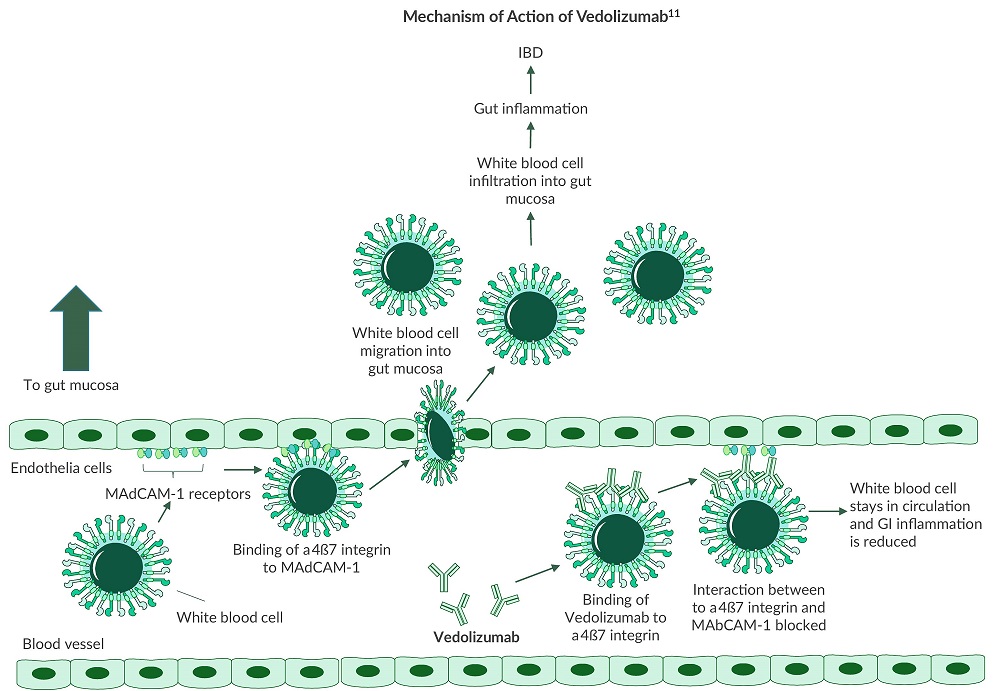
Integrins are transmembrane proteins that enable cells to adhere to their environment and play a role in cell signaling. Research has uncovered three different integrin subunits which are associated with white blood cell migration into the GI tract10:- α2β2
- α4β1
- α4β7
 Several classes of biologics for the treatment of IBD have been developed to suppress inflammation in the GI tract including TNF-alpha blockers, interleukin blockers, and integrin blockers1. These monoclonal antibody biologics successfully use different mechanisms to block the proinflammatory cell signaling of cytokines, interleukins, and proteins in the GI tract.
Several classes of biologics for the treatment of IBD have been developed to suppress inflammation in the GI tract including TNF-alpha blockers, interleukin blockers, and integrin blockers1. These monoclonal antibody biologics successfully use different mechanisms to block the proinflammatory cell signaling of cytokines, interleukins, and proteins in the GI tract.
References
- Neurath. (2017). Current and emerging therapeutic targets for IBD. Nature Reviews Gastroenterology & Hepatology. 14:269–278. doi:10.1038/nrgastro.2016.208.
- Targan et al. (1997). A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N. Engl. J. Med. 337:1029-35.
- Sartor et al. (1994). Cytokines in intestinal inflammation: Pathophysiological and clinical consideration. Gastroenterology. 106:533-39.
- Crohn's & Colitis Foundation. (2014). Biologic Therapies. crohnscolitisfoundation.org.
- Simon et al. (2016). Ustekinumab for the treatment of Crohn's disease: Can it find its niche? Therap Adv Gastroenterol. 2016 Jan;9(1):26–36. PMCID: PMC4699281.
- Crohn's & Colitis Foundation. (2016). FDA Approves STELARA® (Ustekinumab) for Treatment of Moderate to Severe Crohns Disease. crohnscolitisfoundation.org.
- Feagan et al. (2016). Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. doi:1056/NEJMoa1602773.
- Robinson. (2015). IL12Rβ1: The cytokine receptor that we used to know. Cytokine. 2015 Feb;71(2):348-59. PMID: 25516297.
- Koutruba et al. (2010). Review of ustekinumab, an interleukin-12 and interleukin-23 inhibitor used for the treatment of plaque psoriasis. Ther Clin Risk Manag. 2010;6:123–141. PMCID: PMC2857612.
- Cherry et al. (2015). Vedolizumab: An α4β7 integrin antagonist for ulcerative colitis and Crohn's disease. Ther Adv Chronic Dis. 2015 Sep;6(5):224-33. doi: 10.1177/2040622315586970. PMID: 26336591.
- Wyant et al. (2016). An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. Journal of Crohn's and Colitis. 1 December 2016;10(12):1437–1444. https://doi.org/10.1093/ecco-jcc/jjw092.
