Challenges When Treating IBD with Biologics
Monoclonal antibody (mAbs) biologics have been used to treat inflammatory bowel disease (IBD) for 20 years1. Despite the successful use of biologics as therapies for Crohn’s disease (CD) and ulcerative colitis (UC), these diseases remain difficult to manage. As a result, patients and healthcare providers are met with many challenges when treating IBD with biologics such as variable drug response, immunogenicity, subtherapeutic levels, and the overall cost for IBD patients2,3.
Variable Drug Responses to Biologics
Research demonstrates that various responses to the primary biologic can occur when treating IBD. Only an estimated 45% of Crohn’s disease cases treated with TNF-alpha blockers reach clinical response2. Studies also indicate that 50-70% of all CD patients do not achieve complete remission after 6 months of treatment with TNF-alpha blockers4,5. Furthermore, 20% of CD patients treated with TNF-alpha blockers do not respond clinically to their primary biologic which is now referred to as primary non-response (PNR)2. Additionally, research demonstrates that an estimated 35% experience a loss of response (LOR) as biologics treatment progresses2.
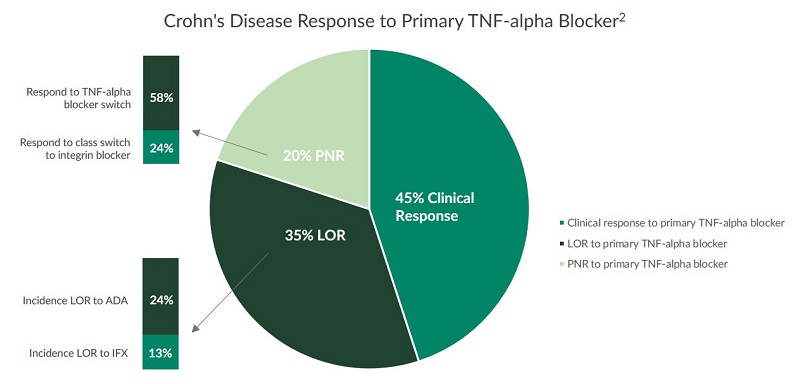
PNR and LOR have proven to be difficult challenges for IBD patients and healthcare providers to overcome. Further research investigating drug responses to biologics is necessary to help improve IBD care. Studies indicate that several factors are associated with primary non-response and loss of response in IBD cases treated with biologics including immunogenicity and subtherapeutic levels of the biologic6.
Immunogenicity of Biologics
Research shows that when a biologic is taken, there is a possibility that the body may mount an immune response against the drug which can involve the formation of anti-drug antibodies (ADAs). This occurrence is known as immunogenicity and can affect both the pharmacokinetic (PK) and pharmacodynamic (PD) properties of biologics6. The development of ADAs may cause adverse events, PNR, LOR, and in rare extreme cases, lead to toxicity6. Many factors such as the design of the biologic, dosage size and administration method, immune system status, and genetics can affect the immunogenicity of biologics6.
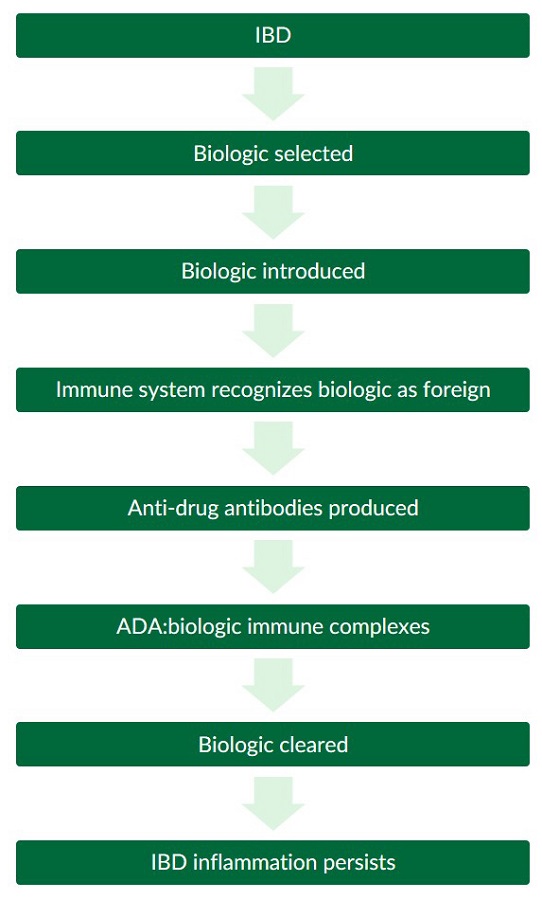
Discovery of Biologics Immunogenicity
The immunogenicity of biologics was first identified in patients treated with infliximab, and prompted the development of other TNF-alpha blockers such as adalimumab7-9. However, at week 4 of a pivotal adalimumab clinical trial, only 21% of patients who had previously been treated with another TNF-alpha blocker (infliximab) achieved remission10. These findings suggest that a proportion of patients can develop resistance to multiple biologics rather than to one single agent10. The identification of immunogenicity reactions caused by both infliximab and adalimumab influenced the development of new classes of biologics to treat IBD11.
Subtherapeutic Levels of Biologics
In 2003, findings from a key study revealed a positive correlation between infliximab concentrations and the duration of response, therefore demonstrating the importance of maintaining optimal infliximab concentrations7. Anti-drug antibody:drug immune complexes may increase the clearance rate of biologics and lead to subtherapeutic levels and loss in efficacy6. However, research also indicates that subtherapeutic levels of biologics with or without the formation of ADAs can lead to LOR and further complicate IBD treatment7. Studies have shown that increasing the dose of infliximab in IBD cases with subtherapeutic levels can lead to improved clinical response12. In order to improve care, though, more research exploring the relationship between pharmacokinetics and efficacy of biologics in IBD cases is still needed13-15.
Cost of IBD Biologics
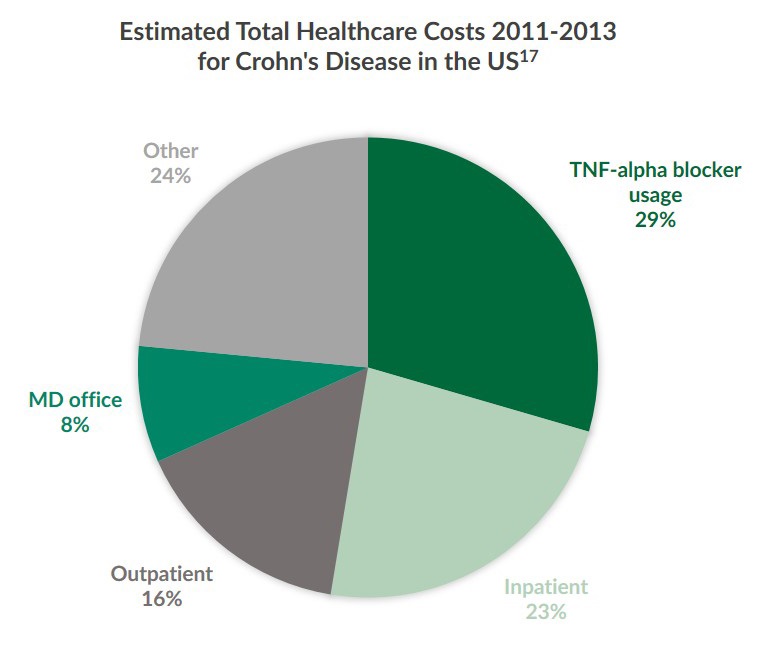
Research indicates that the annual health care cost per member and the usage of biologics for treating IBD is increasing16. Between 2011 and 2013, annual health plans in the U.S. paid an estimated $20,000 USD in IBD care costs per member17. One third of the annual cost for this time was attributed to TNF-alpha blocker biologics alone. Furthermore, healthcare plans during this period paid more as age and the number of comorbidities increased17. Another study reported that the annual cost of IBD treatment rose to $36,051 USD per member in 201516. Investigations also suggest that healthcare providers optimize biologics therapy use in order to reduce the burden of IBD17. However, due to the immunogenicity of biologics, it has been difficult to develop methods to optimize IBD therapies14.
Overcoming Challenges When Treating IBD with Biologics
Although the advent of biologics revolutionized the standard of care for inflammatory bowel diseases, there are still many challenges when treating IBD with biologics2,3. More research on the immunogenicity of biologics can potentially reduce loss of response and prevent primary loss of response to those therapies2. Methods to optimize IBD biologics treatments may reduce the occurrence of subtherapeutic levels, and also increase clinical response2,3. A deeper understanding of both immunogenicity and optimal biologics concentration can lead to reduced treatment costs and improve the quality of life for IBD patients around the globe.
References
Roda et al. (2016). Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016 Jan;7(1):e135. doi:10.1038/ctg.2015.63. PMCID: PMC4737871.
Martelli et al. (2016). Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J. Gastroenterol. 2017 Jan;52(1):19-25. doi: 10.1007/s00535-016-1266-1. PMID: 27665099.
Mowat et al. (2011). Guidelines for the management of inflammatory bowel disease in adults. Gut 2011. doi:10.1136/gut.2010.224154.
St. Clair Jones. (2014). Optimizing therapy for inflammatory bowel disease. Clinical Pharmacist. 6(8).
Smith et al. (2016). Unraveling the Effect of Immunogenicity on the PK/PD, Efficacy, and Safety of Therapeutic Proteins. Journal of Immunology Research. 2016;Article ID 2342187, 9 pages. doi:10.1155/2016/2342187.
Baert et al. (2003). Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N. Engl. J. Med. 348:601-608.
Maser et al. (2006). Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin. Gastroenterol. Hepatol. 4:1248-1254.
Vermeire et al. (2007). Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 56:1226-1231.
Sandborn et al. (2007). Adalimumab for maintenance treatment of Crohn's disease: Results of the CLASSIC II trial. Gut. 56:1232-1239.
Neurath. (2017). Current and emerging therapeutic targets for IBD. Nature Reviews Gastroenterology & Hepatology. 2017;4:269–278. doi:10.1038/nrgastro.2016.208.
Dalal et al. (2015). What to Do When Biologic Agents Are Not Working in Inflammatory Bowel Disease Patients. Gastroenterol Hepatol (N Y). 2015 Oct;11(10):657–665. PMCID: PMC4849518.
Ben-Horin et al. (2015). Optimizing biologic treatment in IBD: Objective measures, but when, how and how often? BMC Gastroenterology. 2015;15:178. DOI 10.1186/s12876-015-0408-x.
Melmed et al. (2016). Appropriateness of Testing for Anti–Tumor Necrosis Factor Agent and Antibody Concentrations, and Interpretation of Results. Clinical Gastroenterology and Hepatology, 14(9):1302–1309. DOI: http://www.cghjournal.org/article/S1542-3565(16)30195-1/fulltexth.
Cheifetz. (2017). Overview of Therapeutic Drug Monitoring of Biologic Agents in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2017 Sep;13(9):556–559. PMCID: PMC5635433.
Yu et al. (2018). Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Ailment Pharmacol Ther. 2018 Feb;47(3):364-370. PMID: 29164650.
Park et al (2016). Health Insurance Paid Costs and Drivers of Costs for Patients With Crohn's Disease in the United States. The American Journal of Gastroenterology. January 2016;111:15-23. doi:10.1038/ajg.2015.207.
